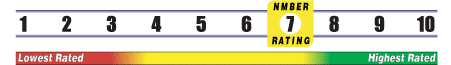
Each tablet contains: PABA 50 mg.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.
Below is general information about the effectiveness of the known ingredients contained in the product PABA. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product PABA. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used topically and appropriately. PABA is FDA approved for topical use and there have not been reports of significant toxicity (266,272).
POSSIBLY SAFE ...when used orally and appropriately (10). PABA is an FDA-approved drug, but some potentially serious side effects have been reported (10).
POSSIBLY UNSAFE ...when used orally in high doses. Doses greater than 12 grams per day have been associated with leukopenia (1061).
CHILDREN: LIKELY SAFE
when used topically and appropriately (266,272).
CHILDREN: POSSIBLY SAFE
when used orally and appropriately (10).
PABA is an FDA-approved drug for use in children, but serious side effects have been reported (10).
CHILDREN: POSSIBLY UNSAFE
when used orally in high doses.
Doses greater than 220 mg/kg/day have been associated with fatal toxic effects (1061).
PREGNANCY AND LACTATION: LIKELY SAFE
when used topically and appropriately (266,272).
There is insufficient reliable information available about the safety of the oral use of PABA during pregnancy and breast-feeding; avoid using.
Below is general information about the interactions of the known ingredients contained in the product PABA. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, concomitant use of PABA and cortisone may increase the effects of cortisone.
Preliminary clinical research shows that PABA decreases the metabolism of cortisone (4488). Dose adjustments of cortisone may be necessary.
|
Theoretically, concomitant use of PABA and dapsone may decrease the effects of dapsone.
|
Theoretically, concomitant use of PABA and sulfonamide antibiotics inhibits the antibacterial effects of sulfonamide.
Sulfonamide antibiotics competitively inhibit folic acid synthesis from PABA. Excess PABA may overcome the folate depleting effect of the sulfonamides (10). Avoid concurrent use.
|
Below is general information about the adverse effects of the known ingredients contained in the product PABA. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally and topically, PABA is generally well tolerated.
Most Common Adverse Effects:
Orally: Anorexia, dyspepsia, fever, nausea, rash, vomiting.
Topically: Dermatitis, paradoxical photosensitivity.
Serious Adverse Effects (Rare):
Orally: Hepatotoxicity.
Dermatologic ...Topically, PABA can cause contact dermatitis and sometimes paradoxical photosensitivity (272).
Gastrointestinal ...Orally, the most commonly reported side effects of PABA are diarrhea, nausea, vomiting, anorexia, dyspepsia, fever, and rash (1074). PABA should be discontinued if adverse effects prevent the patient from eating (10). In one report, up to 25% of patients discontinued PABA due to intolerance of side effects (1074).
Hematologic ...High dose PABA (up to 48 grams per day) can cause decreased white blood count below 4000 mm3 in approximately 30% of patients (1061).
Hepatic ...Liver toxicity, including fatal hepatitis has been reported in patients taking PABA in high doses (12-48 grams per day) (1061,1094). In one case, 12 grams per day for 2 months caused liver toxicity (1094). Increased liver function tests from PABA have also been noted (1084), and jaundice was reported in one case of treatment with PABA (68049). Despite these reports, a study reviewing the charts of 274 patients with scleroderma who were taking the potassium salt form of PABA as treatment found no evidence of hepatotoxicity (1065).
Immunologic ...Orally, PABA can cause allergic reactions including fever and skin rash (1074).
Neurologic/CNS ...Orally, PABA may cause headaches, which resolve upon discontinuation of treatment (1074).
Other ...Death has been reported in 3 children treated with 24 grams of PABA per day for rheumatic fever or arthritis. At autopsy, all had fatty changes in the liver, kidney, and myocardium (1061). Topical PABA and its derivatives have a tendency to discolor clothing due to a photo-oxidative reaction (68025).
