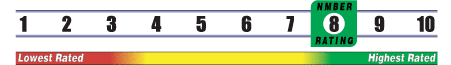
Each softgel capsule contains: Fish Liver Oil 40 mg, providing: Vitamin A (and as vitamin A palmitate) 2400 mcg / 8000 IU. Other Ingredients: Soya Bean Oil, Capsule Shell (gelatine, glycerine), Vitamin A Palmitate, Vitamin E (as mixed tocopherols), Sunflower Oil.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.
Below is general information about the effectiveness of the known ingredients contained in the product Vitamin A with Fish Liver Oils 8,000 IU. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Vitamin A with Fish Liver Oils 8,000 IU. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Doses of 3 grams per day and less can be safely used by most people. Fish oil has Generally Recognized As Safe (GRAS) status in the US (1313,1024,2299,2300,2301,2302,2315,2317,4912,5702)(5705,5706,6394,6399,7368,7369,7380,12921,12922,13011)(13766,14382,16733,17408,17991,17992,66454,89325,89336,89346)(89351,89352,89373,89374,101543,103492,103499,103502,104546,105220)(107180,107181,113220). Although higher doses of fish oil, such as 6 grams daily for up to 1 year, have been used safely (89344), there are some safety concerns about using high doses of fish oil. Some older research suggests that doses greater than 3 grams per day can inhibit blood coagulation and potentially increase bleeding risk (8671,8679,8696,66258,21223,21224). However, the most rigorous research to date shows that short-term doses of fish oil 10 grams daily and long-term doses of 1.5 grams daily for up to 52 weeks do not increase the risk of bleeding or affect coagulation parameters in chronically ill and vulnerable patients (97180). Still, doses greater than 3 grams per day might suppress immune response (1313,7384). Patients should only take high-dose fish oil while under medical supervision.
POSSIBLY SAFE ...when parenteral nutrition supplemented with a lipid emulsion enriched in fish oil is used, short-term. Fish oil or omega-3 fatty acid lipid emulsions, administered intravenously for 1-4 weeks, have been safely used (1004,66042,66421,89323,103497).
POSSIBLY UNSAFE ...when fish oil from dietary sources is consumed in large amounts. Fatty fish can contain significant amounts of toxins such as mercury, polychlorinated biphenyls (PCBs), dioxin, and dioxin-related compounds. Very frequent consumption of contaminated fish can cause adverse effects such as tremor, numbness and tingling, difficulty concentrating, and vision problems. Avoid frequent consumption of swordfish, king mackerel, tilefish (also called golden bass or golden snapper), and farm-raised salmon (12964,12965,12966). There is insufficient reliable information available about the safety of fish oil when used topically.
CHILDREN: POSSIBLY SAFE
when used orally and appropriately (5708,5711,65732,66070).
In adolescents 9 years of age and older, fish oil providing doses of up to 2250 mg omega-3 fatty acids daily have been used with apparent safety for up to 12 weeks (101543). Fish oil used in enteral feeds for up to 9 months has been shown to be safe in infants (13745). Young children should limit dietary consumption to no more than two ounces of fish per week (12967,12968). ...when given as part of parenteral nutrition in infants receiving long-term parenteral nutrition (96118,110340,110346,110352).
CHILDREN: POSSIBLY UNSAFE
when fish oil from dietary sources are consumed in large amounts.
Fatty fish can contain significant amounts of toxins such as mercury, polychlorinated biphenyls (PCBs), dioxin, and dioxin-related compounds. Frequent consumption of contaminated fish can cause brain damage, mental retardation, blindness, and seizures in children. Lower levels can cause more subtle problems such as learning disabilities (12964). Young children should limit consumption to no more than 2 ounces per week of fish (12967,12968).
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally and appropriately.
Intake of fish oil during pregnancy does not appear to adversely affect the fetus or nursing infant (1026,1027,1042,8706,12969,12970,12971,12972,12973,14397)(15015,15162,101540,110338,113217). The adequate intake level of omega-3 fatty acids during pregnancy is 1.4 grams daily; the adequate intake level during lactation is 1.3 grams daily (89377). If possible, people who are trying to become pregnant, as well as those who are pregnant or lactating, should avoid swordfish, king mackerel, and tilefish (also called golden bass or golden snapper), as these may contain high levels of methylmercury. Pregnant individuals should also limit consumption of other fatty fish to 12 ounces, or about 3-4 servings, per week.
PREGNANCY AND LACTATION: POSSIBLY UNSAFE
when fish oil from dietary sources are consumed in large amounts.
Fatty fish can contain significant amounts of toxins such as mercury, polychlorinated biphenyls (PCBs), dioxin, and dioxin-related compounds. If possible, people who are trying to become pregnant, as well as those who are pregnant or lactating, should avoid swordfish, king mackerel, and tilefish (also called golden bass or golden snapper), as these may contain high levels of methylmercury. Pregnant individuals should also limit consumption of other fatty fish to 12 ounces, or about 3-4 servings, per week (12967,12968).
LIKELY SAFE ...when used orally or intramuscularly and appropriately. Vitamin A, as pre-formed vitamin A (retinol or retinyl ester), is safe in adults when taken in doses below the tolerable upper intake level (UL) of 10,000 IU (3000 mcg) per day (7135). Higher doses increase the risk of side effects. There is also growing concern that taking high doses of antioxidants such as vitamin A might do more harm than good. In an analysis of studies, taking vitamin A supplements alone or in combination with other antioxidants is associated with an increased risk of mortality from all causes (15305,90775). Keep in mind that vitamin A is available in two different forms: pre-formed vitamin A (retinol or retinyl ester) and provitamin A (carotenoids). The safety concerns associated with high vitamin A intake occur with pre-formed vitamin A only. Some supplements contain vitamin A in both pre-formed and provitamin A forms. For these supplements, the amount of pre-formed vitamin A should be used to determine if the amount of vitamin A is safe.
POSSIBLY SAFE ...when used topically and appropriately, short-term. Retinol 0.5% has been used on the skin daily for up to 12 weeks with apparent safety. No serious adverse effects have been reported in clinical trials (103671,103680).
POSSIBLY UNSAFE ...when used orally in high doses. Doses higher than the UL of 10,000 IU (3000 mcg) per day of pre-formed vitamin A (retinol or retinyl ester) might increase the risk of side effects (7135). While vitamin A 25,000 IU (as retinyl palmitate) daily for 6 months followed by 10,000 IU daily for 6 months has been used with apparent safety in one clinical trial (95052), prolonged use of excessive doses of vitamin A can cause significant side effects such as hypervitaminosis A. The risk for developing hypervitaminosis A is related to total cumulative dose of vitamin A rather than a specific daily dose (1467,1469). There is also concern that taking high doses of antioxidants such as vitamin A might do more harm than good. In an analysis of studies, taking vitamin A supplements alone or in combination with other antioxidants is associated with an increased risk of mortality from all causes (15305,90775). There is insufficient reliable information available about the safety of using sublingual formulations of vitamin A.
CHILDREN: LIKELY SAFE
when used orally or intramuscularly and appropriately.
The amount of pre-formed vitamin A (retinol or retinyl ester) that is safe depends on age. For children up to 3 years of age, doses less than 2000 IU (600 mcg) per day seem to be safe. For children ages 4 to 8, doses less than 3000 IU (900 mcg) per day seem to be safe. For children ages 9 to 13, doses less than 5667 IU (1700 mcg) per day seem to be safe. For children 14 to 18, doses less than 9333 IU (2800 mcg) per day seem to be safe (7135). Keep in mind that vitamin A is available in two different forms: pre-formed vitamin A (retinol or retinyl ester) and provitamin A (carotenoids). The safety concerns associated with high vitamin A intake occur with pre-formed vitamin A only. Some supplements contain vitamin A in both pre-formed and provitamin A forms. For these supplements, the amount of pre-formed vitamin A should be used to determine if the amount of vitamin A is safe.
CHILDREN: POSSIBLY UNSAFE
when pre-formed vitamin A (retinol or retinyl ester) is used orally in excessive doses.
For children up to 3 years of age, avoid doses greater than 2000 IU (600 mcg) per day. For children ages 4 to 8, avoid doses greater than 3000 IU (900 mcg) per day. For children ages 9 to 13, avoid doses greater than 5667 IU (1700 mcg) per day. For children ages 14 to 18, avoid doses greater than 9333 IU (2800 mcg) per day (7135). Higher doses of vitamin A supplementation have been associated with increased risk of side effects such as pneumonia, bone pain, and diarrhea (319,95051). Long-term supplementation with low to moderate doses on a regular basis can cause severe, but usually reversible, liver damage (11978).
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally or intramuscularly and appropriately.
Vitamin A, as pre-formed vitamin A (retinol or retinyl ester), is safe during pregnancy and lactation when used in doses less than 10,000 IU (3000 mcg) per day (7135,16823,107293). Keep in mind that vitamin A is available in two different forms: pre-formed vitamin A (retinol or retinyl ester) and provitamin A (carotenoids). The safety concerns associated with high vitamin A intake occur with pre-formed vitamin A only. Some supplements contain vitamin A in both pre-formed and provitamin A forms. For these supplements, the amount of pre-formed vitamin A should be used to determine if the amount of vitamin A is safe.
PREGNANCY AND LACTATION: POSSIBLY UNSAFE
when used orally or intramuscularly in excessive doses.
Daily intake of greater than 10,000 IU (3000 mcg) can cause fetal malformations (3066,7135). Excessive dietary intake of vitamin A has also been associated with teratogenicity (11978). The first trimester of pregnancy seems to be the critical period for susceptibility to vitamin A-associated birth defects such as craniofacial abnormalities and abnormalities of the central nervous system (7135). Pregnant patients should monitor their intake of pre-formed vitamin A (retinol or retinyl ester). This form of vitamin A is found in several foods including animal products, some fortified breakfast cereals, and dietary supplements (3066).
Below is general information about the interactions of the known ingredients contained in the product Vitamin A with Fish Liver Oils 8,000 IU. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Fish oil may have antiplatelet effects and may increase the risk of bleeding if used with anticoagulant or antiplatelet drugs. However, evidence is conflicting.
While fish oil may not be a potent inhibitor of platelet function, high doses of fish oil might have antiplatelet effects. Theoretically, concomitant use of fish oil with anticoagulant or antiplatelet drugs may increase the risk of bleeding (8671,8679,8696,13769,21223,21224,66258). However, the most rigorous research shows that short-term doses of fish oil 10 grams daily or long-term doses of 1.5 grams daily for up to 52 weeks does not increase the risk of bleeding or affect coagulation parameters in chronically ill and vulnerable patients (97180). Other controlled research shows that fish oil does not affect platelet function or increase the risk of bleeding (17990,17996,66105,66267,89374,107180). Some research even suggests that perioperative fish oil use decreases bleeding risk (89352). Some research suggests fish oil does not have additive antiplatelet effects when combined with aspirin (13769), but other clinical evidence suggests that adding fish oil to low-dose aspirin treatment increases antiplatelet effects in patients who are aspirin-resistant (21226). Also, some clinical research seems to show that fish oil has additive antiplatelet effects when used with aspirin and clopidogrel compared to aspirin and clopidogrel alone (21225).
|
Theoretically, taking fish oil with antihypertensive drugs might increase the risk of hypotension.
|
Theoretically, taking fish oil with contraceptive drugs might decrease the triglyceride-lowering effects of fish oil.
There is some evidence that contraceptive drugs might interfere with the triglyceride lowering effects of fish oils (8694).
|
Taking fish oil with cyclosporine might increase levels and adverse effects of cyclosporine.
In kidney transplant recipients on a general immunosuppressive regimen, taking omega-3 fatty acids daily seems to increase peak blood levels of cyclosporine when compared with placebo. This increase was as much as 20% after one month. However, the area under the curve was not significantly affected (66472).
|
Theoretically, taking fish oil with orlistat might decrease the absorption of fish oil fatty acids.
Orlistat binds lipase in the gastrointestinal tract and reduces fat absorption. Theoretically, taking fish oil with orlistat might decrease absorption of fish oil fatty acids. To avoid this potential interaction, recommend separating administration of orlistat and fish oil by at least 2 hours.
|
Theoretically, taking fish oil with platinum agents can cause resistance to platinum agents, potentially decreasing their effectiveness.
Platinum-induced fatty acids (PIFAs) are fatty acids secreted from human and mouse stem cells when exposed to platinum-based chemotherapy. Animal research suggests that PIFAs cause resistance to chemotherapy by stimulating lysophospholipid production in the spleen, which interferes with the DNA damage caused by certain chemotherapy drugs (92076). One PIFA, known as 16:4(n-3), has been found in both raw fish and some commercially available fish oil products. Mackerel and herring have high PIFA concentrations, while salmon and tuna have low PIFA concentrations. Levels of PIFA in commercial fish oil products ranged from 0.2- 5.7 microMol. Animal research shows that PIFA-containing fish oil products cause resistance to cisplatin, fluorouracil, irinotecan, and oxaliplatin (91250,92075). It is unclear if all commercially available fish oil products contain PIFAs. Additionally, it is argued that levels of PIFA found in some fish oil products are too low to be of clinical concern. Furthermore, a lack of chemotherapy resistance in countries with high fish intake, such as Greenland, Japan, and Norway, suggest that this interaction may not be clinically significant (91288,91289).
|
Taking fish oil with sirolimus might increase levels and adverse effects of sirolimus.
Pharmacokinetic research shows that omega-3 fatty acids increase exposure to sirolimus in kidney transplant patients on a calcineurin inhibitor-free immunosuppressive regimen. A 25% dose reduction in sirolimus was required to keep patients within the expected trough-concentration window (105232). Researchers hypothesize that this may be due to inhibition of cytochrome P450 3A4 (CYP3A4) by fish oil, although this has not been confirmed in clinical research.
|
Taking fish oil with tacrolimus might increase levels and adverse effects of tacrolimus.
In a small group of patients, taking fish oil 2.6 grams (Omacor) daily for 4 weeks increased the 8-hour area under the curve of tacrolimus by 25% when compared with baseline. Peak levels were increased by approximately 22% (105212). Researchers hypothesize that this may be due either to an increase in bioavailability or to inhibition of cytochrome P450 3A4 (CYP3A4) by fish oil, although this has not been confirmed in clinical research.
|
Fish oil may have antiplatelet effects and might increase the risk of bleeding if used with warfarin.
|
Theoretically, taking high doses of vitamin A in combination with other potentially hepatotoxic drugs might increase the risk of liver disease.
|
Concomitant use of retinoids with vitamin A supplements might produce supratherapeutic vitamin A levels.
Retinoids, which are vitamin A derivatives, could have additive toxic effects when taken with vitamin A supplements (3046).
|
Theoretically, taking tetracycline antibiotics with high doses of vitamin A can increase the risk of pseudotumor cerebri.
Benign intracranial hypertension (pseudotumor cerebri) can occur with tetracyclines and with acute or chronic vitamin A toxicity. Case reports suggest that taking tetracyclines and vitamin A concurrently can increase the risk of this condition (10545,10546,10547). Avoid high doses of vitamin A in people taking tetracyclines chronically.
|
Theoretically, high doses of vitamin A could increase the risk of bleeding with warfarin.
Vitamin A toxicity is associated with hemorrhage and hypoprothrombinemia, possibly due to vitamin K antagonism (505). Advise patients taking warfarin to avoid doses of vitamin A above the tolerable upper intake level of 10,000 IU/day for adults.
|
Below is general information about the adverse effects of the known ingredients contained in the product Vitamin A with Fish Liver Oils 8,000 IU. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally and parenterally, fish oil is generally well-tolerated.
Topically, no adverse effects have been reported. However, a thorough evaluation of safety outcomes has not been conducted.
Most Common Adverse Effects:
Orally: Abdominal pain, bad breath, fishy aftertaste, heartburn, increased low-density lipoprotein (LDL) cholesterol levels, loose stools, nausea, and rash.
Serious Adverse Effects (Rare):
Orally: Atrial fibrillation. When taken in doses of 3 grams or more daily, there are rare reports of increased risk of bleeding and stroke, as well as immune suppression.
Cardiovascular
...Orally, fish oil supplements in doses of 3-10 grams daily can cause a dose-dependent increase in low-density lipoprotein (LDL) cholesterol levels in some people (2299,2318,8678,8698,15734,15735,48120,65729) by increasing the size of LDL particles (9771).
Therefore, LDL levels should be monitored in people who take fish oil supplements (15734). But fish oil doesn't seem to cause development of atherosclerosis, despite earlier concerns that polyunsaturated fatty acids, such as omega-3 fatty acids, might increase the oxidation of LDL (1011,2323,7165,7366,8695,8700,9771).
There is concern that fish oil supplements may be associated with an increased risk for atrial fibrillation (AF), particularly in patients with or at risk for cardiovascular disease (CVD). In one large clinical study (the STRENGTH trial), taking a prescription fish oil product (Epanova) 4 grams daily for up to 5 years was associated with an increased risk for AF, with a number needed to harm of 114 when compared with a corn oil control. The patients in this study were considered to be at high risk for future CVD (103491).
In a secondary analysis of the Omega-3 fatty acids in Elderly patients with Myocardial Infarction (OMEMI) trial, adults aged 70-82 years with recent myocardial infarction supplementing with 1.8 grams daily of n-3 PUFA (EPA/DHA) for 24 months had a 90% increased risk of developing AF or micro-AF, which is characterized by short, AF-like activity lasting less than 30 seconds. Changes in serum EPA levels seem to mediate this risk, with higher serum EPA levels predictive of an increased risk of AF and intermediate serum EPA levels predictive of an increased risk of micro-AF (112469).
Meta-analyses of randomized controlled trials show that taking omega-3 fatty acid supplements increases the incidence rate ratio for AF by up to 37% when compared with placebo, with an increased incidence rate associated with doses of more than 1 gram daily. One study in these analyses used eicosapentaenoic acid (EPA) alone as purified icosapent ethyl (106075,107171,107181).
In 2023, the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency issued a public statement on the use of omega-3-acid ethyl esters, concluding that exposure to omega-3-acid ethyl esters for the treatment of hypertriglyceridemia is associated with a dose-dependent increased risk of AF in patients with established cardiovascular disease or risk factors for cardiovascular disease when compared with placebo. The highest risk of AF is associated with a dose of 4 grams daily. If AF occurs, treatment should be permanently discontinued (112467).
Dermatologic
...Orally, skin rashes, itching, and skin irritation have been reported (66498,66492,66488,89369,104551).
A case of severe tissue inflammation and breast tissue necrosis has been reported for a female who injected fish oil from capsules subcutaneously for breast augmentation (89371).
Gastrointestinal
...Orally, most adverse effects of omega-3 fatty acids are gastrointestinal in nature (12915,65599,65728,65770,65780,65904,66061,66104,66164,66488)(89347,89357,103491,103551,107172).
Gastrointestinal upset is common with the use of fish oil supplements, occurring in up to 5% of patients in clinical trials, with nausea in up to 1.5% of patients (2307,16733,65599,65732,65762,65830,65886,65925,65974)(66020,66042,66083,66104,66130,66164,66169,66358,66370,66488)(66494,66498,89347,103491,104551,110353). Diarrhea and loose stools may also occur (6257,10871,65599,65648,65830,65935,66042,66061,66104,66119)(66130,66173,66492,89347,89359,103491,104551,107172,110353), with potentially severe diarrhea at very high doses (12986,16901,66093,66356). There have also been reports of increased burping (17963,65648,65729,65770,65830,66020,66042,66077,66164,66169)(66492,66494,104551), acid reflux, heartburn (104551,110338), indigestion (65566,65830,66061,66104,66173,89359), abdominal bloating (66083,66104), abdominal or gastrointestinal pain or discomfort (8680,17996,66119,66492,103491), anorexia (62390,89359), flatulence (66492), constipation (16901), nausea and vomiting (65728,65830,66104,66130,66488,103491,104551), steatorrhea (66119,66354,66356), fishy hiccups (66488), metallic taste (66488), and a fishy breath odor and aftertaste (16901,65648,65729,65762,66020,66042,66061,66077,66083,66104)(66130,66164,66172,66173,66424,66488,66494,89369,104551,107172). Also, some preliminary evidence suggests that increased serum levels of omega-3 fatty acids, especially DHA, might increase the risk for atrophic gastritis (8709).
Gastrointestinal side effects may be minimized if fish oils are taken with meals and if doses are started low and gradually increased. Taking supplements with meals or freezing them seems to help decrease these side effects for some patients (12975). Enteric coated fish products might also help reduce side effects (6258).
Hematologic
...Orally, 3 grams or greater of omega-3 fatty acids daily may inhibit platelet aggregation and increase the risk of bleeding; however, there is little evidence of statistically significant bleeding risk at lower doses (1313,8699,66500,66501,66334,101543).
Very large intakes of fish oil or omega-3 fatty acids (more than 46 grams per day) may increase the risk of ischemic or hemorrhagic (bleeding) stroke (7603,66502).
A case of hemolytic anemia has been reported for an infant with short bowel syndrome who developed liver disease from total parenteral nutrition (TPN) and was switched to a specific TPN with fish oil (Omegaven, Fresenius-Kabi, Graz, Austria) instead. After stopping the fish oil-TPN, the anemia was reversed suggesting that parenteral fish oil might cause hemolytic anemia (66022).
Hepatic ...Orally, mild elevations in liver function tests, such as alanine aminotransferase and aspartate aminotransferase, have been reported rarely (66353,113218).
Immunologic
...A case of anaphylaxis following ingestion of a fish oil capsule has been reported for a female patient with a history of allergy to crab.
The patient was treated successfully with epinephrine, but had several recurrences of stridor over the next 2 days (89378).
There is also some evidence that fish oil in doses greater than 3 grams per day might adversely affect immune function. Fish oil appears to suppress T- and B-cell function and to reduce the production of cytokines, which might be detrimental to elderly people and people with suppressed immune function such as patients with human immunodeficiency virus (HIV) infection (1313,7383,7384).
Neurologic/CNS ...Orally, fish oil may cause headache, dizziness, and inability to sleep (65599,65648,89359,103491,104551). Also, restlessness and formication have been reported in less than 1% of studied cases of patients taking fish oil (66498).
Oncologic ...There is some concern that high fish intake may increase the risk for certain types of cancer. One large epidemiological study has found that dietary intake of fish oil from fatty fish twice a week or more is associated with a 16% increased risk of breast cancer when compared with eating fatty fish less than twice weekly (107175). Additionally, an analysis of the NIH-AARP Diet and Health Study, a prospective cohort study in over 491,000 older adults, has found that total intake of fish is associated with increased melanoma risk over a median follow-up of 15.5 years. When the lowest and highest quintiles of intake are compared, there is a 22% increase in the risk for malignant melanoma and a 28% increase in the risk for melanoma in situ. In sub-group analyses, all melanoma incidence is positively associated with tuna intake or non-fried fish intake, but malignant melanoma incidence is inversely associated with fried fish intake (108509). It was suggested that the positive associations could be due to contaminants in fish such as polychlorinated biphenyls, dioxins, arsenic, and mercury.
Pulmonary/Respiratory ...Orally, fish oil has been reported to cause nasopharyngitis and upper respiratory tract infections in 3. 3% of patients in one clinical trial (65798). Exacerbation of asthma and apnea have been reported for patients using fish oil (1040,66061,66119,66354).
Other ...Fish oil can contribute to caloric intake and may cause weight gain if used long-term. One gram of fat or oil provides 9 kcal (6871). Fish oil capsules containing 500 mg omega-3 fatty acids in 1 gram of oil would supply about 13.5 kcal per capsule (6871,6874). Fish oil supplements also contain cholesterol in amounts from 1-6 mg per gram of fish oil (3022,6871).
General
...Orally, vitamin A is generally well-tolerated at doses below the tolerable upper intake level (UL).
Serious Adverse Effects (Rare):
Orally: In very high doses, vitamin A can cause pseudotumor cerebri, pain, liver toxicity, coma, and even death.
Dermatologic ...Chronic oral use of large amounts of vitamin A causes symptoms of vitamin A toxicity including dry skin and lips; cracking, scaling, and itchy skin; skin redness and rash; hyperpigmentation; shiny skin, and massive skin peeling (7135,95051). Hypervitaminosis A can cause brittle nails, cheilitis, gingivitis, and hair loss (15,95051). Adverse effects from a single ingestion of a large dose of vitamin A is more common in young children than adults (15). In children, approximately 25,000 IU/kg can cause skin redness and generalized peeling of the skin a few days later and may last for several weeks (15).
Gastrointestinal ...There is some evidence that oral vitamin A supplementation might increase the risk of diarrhea in children. Although vitamin A can prevent diarrhea and reduce mortality in malnourished children, doses as low as 10,000 IU weekly for 40 weeks have been associated with diarrhea in well-nourished children (319). Diarrhea (82326,82389), nausea (7135,100329), abdominal pain (95051), abdominal fullness (100329), and vomiting (7135,82559,95051,95055,109755) have been reported following use of large doses of oral vitamin A. Adverse effects from a single ingestion of a large dose of vitamin A is more common in young children than adults (15). In children, approximately 25,000 IU/kg can cause vomiting and diarrhea (15). Chronic use of large amounts of vitamin A causes symptoms of vitamin A toxicity including anorexia, abdominal discomfort, and nausea and vomiting (7135).
Genitourinary ...Hypervitaminosis A can cause reduced menstrual flow (15). Intravaginally, all-trans retinoic acid can cause vaginal discharge, itching, irritation, and burning (9199).
Hematologic ...Hypervitaminosis A can cause spider angiomas, anemia, leukopenia, leukocytosis, and thrombocytopenia (15,95051).
Hepatic ...Since the liver is the main storage site for vitamin A, hypervitaminosis A can cause hepatotoxicity, with elevated liver enzymes such as alanine aminotransferase (ALT, formerly SGPT) and aspartate aminotransferase (AST, formerly SGOT), as well as fibrosis, cirrhosis, hepatomegaly, portal hypertension, and death (6377,7135,95051).
Musculoskeletal
...Vitamin A can increase the risk for osteoporosis and fractures.
Observational research has found that chronic, high intake of vitamin A 10,000 IU or more per day is associated with an increased risk of osteoporosis and hip fracture in postmenopausal adults, as well as overall risk of fracture in middle-aged males (7712,7713,9190). A meta-analysis of these and other large observational studies shows that high dietary intake of vitamin A or retinol is associated with a 23% to 29% greater risk of hip fracture when compared with low dietary intake (107294). High serum levels of vitamin A as retinol also increase the risk of fracture in males. Males with high serum retinol levels are seven times more likely to fracture a hip than those with lower serum retinol levels (9190). Vitamin A damage to bone can occur subclinically, without signs or symptoms of hypervitaminosis A. Some researchers are concerned that consumption of vitamin A fortified foods such as margarine and low-fat dairy products in addition to vitamin A or multivitamin supplements might cause excessive serum retinol levels. Older people have higher levels of vitamin A and might be at increased risk for vitamin A-induced osteoporosis.
Vitamin A's effects on bone resorption could lead to hypercalcemia (95051).
Hypervitaminosis can cause slow growth, premature epiphyseal closure, painful hyperostosis of the long bones, general joint pain, osteosclerosis, muscle pain, and calcium loss from the bones (15,95051). One child experienced severe bone pain after taking vitamin A 600,000 IU daily for more than 3 months (95051). Vitamin A was discontinued and symptoms lessened over a period of 2 weeks. The patient made a full recovery 2 months later.
Neurologic/CNS
...Orally, adverse effects from a single large dose of vitamin A are more common in young children than adults (15).
Headache, increased cerebrospinal fluid pressure, vertigo, and blurred vision have been reported following an acute oral dose of vitamin A 500,000 IU (7135). In children, approximately 25,000 IU/kg can cause headache, irritability, drowsiness, dizziness, delirium, and coma (15). Chronic use of large amounts of vitamin A causes symptoms of vitamin A toxicity including fatigue, malaise, lethargy, and irritability (7135).
There are reports of bulging of the anterior fontanelle associated with an acute high oral dose of vitamin A in infants (7135,90784,95053,95054). In children, approximately 25,000 IU/kg can cause increased intracranial pressure with bulging fontanelles in infants (15). Also, muscular incoordination has been reported following short-term high doses of vitamin A (7135).
A case of intracranial hypertension involving diffuse headaches and brief loss of vision has been reported secondary to topical use of vitamin A. The patient was using over-the-counter vitamin A preparations twice daily including Avotin 0.05% cream, Retin-A gel 0.01%, and Isotrexin gel containing isotretinoin 0.05% and erythromycin 2%, for treatment of facial acne. Upon exam, the patient was noted to have bilateral optic disc edema. The patient discontinued use of topical vitamin A products. Two months later, the patient reported decreased headaches and an improvement in bilateral optic disc edema was seen (95056).
Ocular/Otic ...In children, oral vitamin A approximately 25,000 IU/kg can cause swelling of the optic disk, bulging eyeballs, and visual disturbances (15). Adverse effects from a single ingestion of a large dose of vitamin A are more common in young children than adults (15).
Oncologic ...There is concern that high intake of vitamin A might increase some forms of cancer. Population research suggests high vitamin A intake might increase the risk of gastric carcinoma (9194).
Psychiatric ...Chronic oral use of large amounts of vitamin A causes symptoms of vitamin A toxicity, which can include symptoms that mimic severe depression or schizophrenic disorder (7135).
Pulmonary/Respiratory ...There is some evidence that oral vitamin A supplementation might increase the risk of pneumonia and diarrhea in children. Although vitamin A can prevent diarrhea and reduce mortality in malnourished children, doses as low as 10,000 IU weekly for 40 weeks have been associated with pneumonia and diarrhea in well-nourished children (319). In preschool children, high-dose vitamin A also increases the risk of respiratory infection (82288).
Other ...Chronic use of large amounts of vitamin A (>25,000 IU daily for more than 6 years or 100,000 IU daily for more than 6 months) can cause symptoms of vitamin A toxicity including mild fever and excessive sweating (7135). High intakes of vitamin A may result in a failure to gain weight normally in children and weight loss in adults (15).
