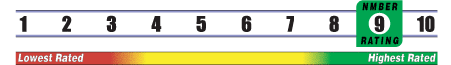
Each capsule contains: 2-Amino-2-Deoxy-Beta-D-Glucopyranose 500 mg. Other Ingredients: Calcium Phosphate, Hypromellose, Vegetable Grade Magnesium Stearate, Silica Gel.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.

In 2004, Canada began regulating natural medicines as a category of products separate from foods or drugs. These products are officially recognized as "Natural Health Products." These products include vitamins, minerals, herbal preparations, homeopathic products, probiotics, fatty acids, amino acids, and other naturally derived supplements.
In order to be marketed in Canada, natural health products must be licensed. In order to be licensed in Canada, manufacturers must submit applications to Health Canada including information about uses, formulation, dosing, safety, and efficacy.
Products can be licensed based on several criteria. Some products are licensed based on historical or traditional uses. For example, if an herbal product has a history of traditional use, then that product may be acceptable for licensure. In this case, no reliable scientific evidence is required for approval.
For products with non-traditional uses, some level of scientific evidence may be required to support claimed uses. However, a high level of evidence is not necessarily required. Acceptable sources of evidence include at least one well-designed, randomized, controlled trial; well-designed, non-randomized trials; cohort and case control studies; or expert opinion reports.
Finished products licensed by Health Canada must be manufactured according to Good Manufacturing Practices (GMPs) as outlined by Health Canada.
Below is general information about the effectiveness of the known ingredients contained in the product Sulfate De Glucosamine 500 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Sulfate De Glucosamine 500 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when glucosamine sulfate is used orally and appropriately. Glucosamine sulfate has been used safely in multiple clinical trials at a dose of 1000-1500 mg daily for 4 weeks to 3 years (2604,7026,8942,11340,12461)(14305,16717,89558,89567,94380,94382,95785).
POSSIBLY SAFE ...when glucosamine hydrochloride is used orally and appropriately. Glucosamine hydrochloride has been used with apparent safety at a dose of 1400-1600 mg daily for up to 2 years (4237,13579,14809,18344,42477,89516,89519,95784). Glucosamine hydrochloride 2 grams daily has also been used with apparent safety for up to 3 weeks (103281). ...when N-acetyl glucosamine is used orally and appropriately. N-acetyl glucosamine 100 mg daily has been used with apparent safety for up to 24 weeks (95795). ...when N-acetyl glucosamine is applied topically and appropriately. A 2% N-acetyl glucosamine cream has been safely used for up to 10 weeks (92721). ...when N-acetyl glucosamine is used rectally and appropriately. N-acetyl glucosamine 3-4 grams daily in 2 divided doses has been safely used (10234). ...when glucosamine sulfate is used intramuscularly and appropriately, short-term. Intramuscular glucosamine sulfate seems to be well tolerated when given twice weekly for up to 6 weeks (2605).
PREGNANCY AND LACTATION:
Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product Sulfate De Glucosamine 500 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Acetaminophen might interfere with the activity of glucosamine sulfate by interacting with the sulfate portion.
Anecdotal reports suggest that adding glucosamine to an acetaminophen regimen might decrease pain control in patients with osteoarthritis (14806). Some research suggests that the sulfate portion of glucosamine sulfate might contribute to its effect in osteoarthritis. Since acetaminophen metabolism requires sulfur and reduces serum sulfate concentrations, acetaminophen could theoretically interfere with the action of glucosamine sulfate. Conversely, the administration of sulfate could theoretically decrease the effectiveness of acetaminophen in sulfate-deficient people by increasing its clearance (10313).
|
Despite initial concerns, it is unlikely that glucosamine will interfere with the effects of antidiabetes drugs.
In vitro and animal research has suggested that glucosamine might increase insulin resistance or decrease insulin production (371,372,3406,18342,18343). This has raised concerns that taking glucosamine might worsen diabetes and decrease the effectiveness of diabetes drugs. However, clinical research suggests that glucosamine does not have adverse effects on blood glucose or glycated hemoglobin (HbA1C) in healthy, obese, or type 2 diabetes patients (7026,7075,8942,10311,10317,15111).
|
Theoretically glucosamine may induce resistance to topoisomerase II inhibitors.
In vitro research suggests that glucosamine might induce resistance to etoposide (VP16, VePesid) and doxorubicin (Adriamycin) by reducing inhibition of topoisomerase II, an enzyme required for DNA replication in tumor cells (7639). This effect has not been reported in humans.
|
Glucosamine might increase the anticoagulant effects of warfarin and increase the risk of bruising and bleeding.
In two individual case reports, glucosamine/chondroitin combinations were associated with a significant increase in international normalized ratio (INR) in patients previously stabilized on warfarin (11389,16130). In one case, the increase in INR occurred only after tripling the dose of a glucosamine/chondroitin supplement from 500 mg/400 mg daily to 1500/1200 mg daily (16130). Additionally, 20 voluntary case reports to the U.S. Food & Drug Administration (FDA) have linked glucosamine plus chondroitin with increased INR, bruising, and bleeding in patients who were also taking warfarin (16130). There have also been 20 additional case reports to the World Health Organization (WHO) that link glucosamine alone to increased INR in patients taking warfarin (16131). The mechanism of this interaction is unclear. Glucosamine is a small component of heparin, but is not thought to have anticoagulant activity; however, animal research suggests that it might have antiplatelet activity (16131).
|
Below is general information about the adverse effects of the known ingredients contained in the product Sulfate De Glucosamine 500 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, all forms of glucosamine seem to be well tolerated.
Topically and rectally, N-acetyl glucosamine also seems to be well tolerated. Intramuscularly, glucosamine sulfate seems to be well tolerated. However, a thorough evaluation of safety outcomes has not been conducted for non-oral routes of administration.
Most Common Adverse Effects:
Orally: Bloating, constipation, cramps, diarrhea, heartburn, nausea.
Serious Adverse Effects (Rare):
Orally: There have been rare reports of severe allergic reactions and hepatotoxicity.
Cardiovascular
...One case of mesenteric occlusion in a clinical trial was considered possibly related to use of oral glucosamine hydrochloride and chondroitin sulfate (89520).
Some observational research has found that glucosamine use in patients with osteoarthritis is associated with a higher risk of cardiovascular disease (CVD) events when compared with non-use (109642). However, glucosamine users tended to be older, have multiple comorbidities, and be on antihyperlipidemic or antiplatelet therapy. Furthermore, other observational research in healthy adults has found that glucosamine use is associated with a reduced risk of fatal and non-fatal CVD events (99682). Higher quality, prospective research is needed to clarify the relationship, if any, between glucosamine and CVD risk.
Dermatologic ...Orally, glucosamine might cause skin reactions, including itching, rash, and erythema (2608,20084,89567,110628,113636). Also, fingernail and toenail toughening, with an increased rate of growth, has been reported (89572). Topically, N-acetyl glucosamine 2% with niacinamide 4% cream might cause rare skin reactions (92721). Photosensitization that was reproducible with re-challenge was reported in a case report of an individual using glucosamine (form unknown) and chondroitin (10408).
Endocrine ...Orally, glucosamine does not seem to impact blood glucose. Preliminary research and anecdotal reports have found that various forms of glucosamine might increase insulin resistance or decrease insulin production, increasing fasting plasma glucose levels (22,371,372,1203,3406,5059,7637,14810). This has raised concerns that taking glucosamine sulfate might worsen diabetes and decrease the effectiveness of diabetes drugs. However, clinical research suggests that various forms of glucosamine do not have adverse effects on blood glucose or glycated hemoglobin (HbA1C) in healthy, obese, patients with type 2 diabetes or impaired glucose tolerance (7026,7075,7638,8942,10311,10317,12107,14808,15111,89563).
Gastrointestinal ...Orally, glucosamine has been associated with gastrointestinal problems, including epigastric and abdominal pain, cramps, heartburn, diarrhea, nausea, dyspepsia, vomiting, constipation, and flatulence (1520,2608,16717,20084,20104,20105,89561,89562,89567,89568)(108897,110628,111647,113636). In older persons, use of glucosamine sulfate is associated with oral dryness (89564). In a clinical trial, a case of Helicobacter pylori gastritis was considered probably related to the use of glucosamine hydrochloride (89516).
Hepatic ...Although relatively uncommon, combinations of glucosamine and chondroitin sulfate have been associated with acute liver injury that mimics autoimmune hepatitis. Of 151 patients at an outpatient clinic for liver diseases, 23 acknowledged use of products containing glucosamine (form unspecified) and/or chondroitin. However, only 2 cases had an apparent relationship between transaminase elevation and the use of recommended doses of glucosamine and chondroitin sulfate. Aminotransferase levels, which were increased by four- to seven-fold, returned to normal following discontinuation of treatment (89515). In another case, a 65-year-old male presented to the hospital with signs and symptoms of drug-induced autoimmune hepatitis. The patient had used Condrosulf, containing chondroitin sulfate, for two years, followed by Vita Mobility Complex, containing chondroitin sulfate and glucosamine sulfate, for 8 weeks. The patient required maintenance treatment with azathioprine to remain in remission (89518). A case of acute cholestatic hepatitis due to Glucosamine Forte, which contains glucosamine hydrochloride, chondroitin sulfate, Devil's claw, and shark cartilage, has been reported (89522). It is unclear whether these adverse events were related to glucosamine, other ingredients, or the combination.
Immunologic ...There is some concern that glucosamine products might cause allergic reactions in sensitive individuals. One review of glucosamine-related adverse events in Australia found that 72% of all reports involved hypersensitivity reactions. Of these reactions, 35% were mild, including pruritis, urticaria, and lip edema, 49% were moderate, including dyspnea, and 16% were severe, including gait disturbance, somnolence, and hypotension. Anaphylaxis was reported in 1.5% of cases (102115). Also, in one clinical trial, a single patient developed allergic dermatitis considered to be likely due to glucosamine hydrochloride (89516). Glucosamine is derived from the exoskeletons of shrimp, lobster, and crabs. However, it is unclear if these adverse reactions were due to a shellfish sensitivity or general atopy. Additionally, shellfish allergies are caused by IgE antibodies to antigens in the meat of shellfish, not to antigens in the exoskeleton. Regardless, it is possible that some glucosamine products might be contaminated by this allergen during production (102115).
Neurologic/CNS ...Orally, glucosamine has been reported to cause drowsiness and headache (2608,89561,113636). Glucosamine plus chondroitin combination products that also contain manganese (e.g., CosaminDS) should always be taken according to product directions. When taken at doses slightly higher than the recommended dose, these products can sometimes supply greater than the tolerable upper limit (UL) for manganese which is 11 mg/day. Ingestion of more than 11 mg/day of manganese might cause significant central nervous system toxicity (7135).
Ocular/Otic ...In older persons, use of glucosamine sulfate has been associated with ocular dryness (89564). Increased intraocular pressure has occurred with glucosamine sulfate supplementation (89573,112460). Data from the FDA MedWatch adverse event reporting system shows that 0.21% of subjects taking glucosamine reported glaucoma, which is significantly greater than the 0.08% of subjects who reported glaucoma while using any other drug (112460).
Pulmonary/Respiratory ...Cases of asthma exacerbations associated with the use of glucosamine (form unknown)-chondroitin products have been reported (10002).
Renal ...Anecdotal reports have associated glucosamine with nephrotoxicity signals such as modestly elevated creatine phosphokinase and 1+ to 2+ proteinuria, but changes in kidney function have not been reported in long-term studies (7026,8942,10408,10409). It was also noted that effects may have been due to other concurrent medications or impurities in glucosamine-chondroitin products. Cases of acute interstitial nephritis induced by glucosamine (form unknown) have also been reported (89523).
Other ...There has been concern that glucosamine might increase the risk of metabolic disturbances resulting in increased cholesterol levels and blood pressure. However, glucosamine does not appear to increase the risk of these adverse effects. Taking glucosamine sulfate for up to 3 years does not significantly increase blood glucose or lipid levels, or cause any other disturbances in metabolism (7026,7075,8942,10311,10317).
