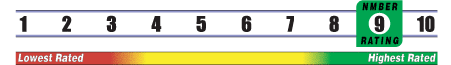
Each tablet contains: Biotin 300 mcg. Other Ingredients: Croscarmellose Sodium, Dibasic Calcium Phosphate, Magnesium Stearate, Microcrystalline Cellulose, Silicon Dioxide, Stearic Acid.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.

In 2004, Canada began regulating natural medicines as a category of products separate from foods or drugs. These products are officially recognized as "Natural Health Products." These products include vitamins, minerals, herbal preparations, homeopathic products, probiotics, fatty acids, amino acids, and other naturally derived supplements.
In order to be marketed in Canada, natural health products must be licensed. In order to be licensed in Canada, manufacturers must submit applications to Health Canada including information about uses, formulation, dosing, safety, and efficacy.
Products can be licensed based on several criteria. Some products are licensed based on historical or traditional uses. For example, if an herbal product has a history of traditional use, then that product may be acceptable for licensure. In this case, no reliable scientific evidence is required for approval.
For products with non-traditional uses, some level of scientific evidence may be required to support claimed uses. However, a high level of evidence is not necessarily required. Acceptable sources of evidence include at least one well-designed, randomized, controlled trial; well-designed, non-randomized trials; cohort and case control studies; or expert opinion reports.
Finished products licensed by Health Canada must be manufactured according to Good Manufacturing Practices (GMPs) as outlined by Health Canada.
Below is general information about the effectiveness of the known ingredients contained in the product Biotin 300 mcg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Biotin 300 mcg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Biotin has been safely used in doses up to 300 mg daily for up to 6 months. A tolerable upper intake level (UL) has not been established (1900,6243,95662,102965). ...when applied topically as cosmetic products at concentrations of 0.0001% to 0.6% biotin (19344).
POSSIBLY SAFE ...when used intramuscularly and appropriately (8468,111366).
CHILDREN: LIKELY SAFE
when used orally and appropriately.
Biotin has been safely used at adequate intake doses of 5-25 mcg daily for up to 6 months (173,6243,19347,19348,111365). A tolerable upper intake level (UL) has not been established.
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally and appropriately.
Biotin has been safely used at the adequate intake (AI) dose of 30 mcg daily during pregnancy and 35 mcg daily during lactation. It has also been used in supplemental doses of up to 300 mcg daily (6243,7878). A tolerable upper intake level (UL) has not been established.
Below is general information about the interactions of the known ingredients contained in the product Biotin 300 mcg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Below is general information about the adverse effects of the known ingredients contained in the product Biotin 300 mcg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally and topically, biotin is generally well tolerated.
Most Common Adverse Effects: None.
Gastrointestinal ...Orally, high-dose biotin has been rarely associated with mild diarrhea. Transient mild diarrhea was reported by 2 patients taking biotin 300 mg daily (95662).
Pulmonary/Respiratory ...In one case report in France, a 76-year-old female frequent traveler developed eosinophilic pleuropericarditis after taking biotin 10 mg and pantothenic acid 300 mg daily for 2 months. She had also been taking trimetazidine for 6 years (3914). Whether eosinophilia in this case was related to biotin, pantothenic acid, other substances, or patient-specific conditions is unknown. There have been no other similar reports.
