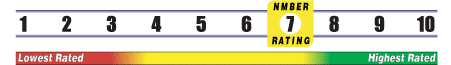
| Ingredients | Amount Per Serving |
|---|---|
|
Cholesterol
|
<5 mg |
|
(from Cod Liver Oil)
(Vitamin A (Form: from Cod Liver Oil) )
|
1250 IU |
|
(Cod Liver Oil)
(Vitamin D (Form: from Cod Liver Oil) )
|
135 IU |
| 415 mg | |
|
(C20:5n-3, EPA)
|
34 mg |
|
(C22:6n-3, DHA)
|
32 mg |
Cod Liver Oil, Gelatin, Glycerin
Below is general information about the effectiveness of the known ingredients contained in the product Natural Cod Liver Oil. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Natural Cod Liver Oil. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. In clinical studies of cod liver oil in adults, a dose of 10 mL daily has been used safely for up to 24 weeks (3398), a dose of 15 mL daily has been used safely for 16 weeks (7360), and doses of 20 mL to 30 mL daily have been used with apparent safety for between 2 weeks and 8 weeks (3399,4026,10083,94758,94759,94754). Cod liver oil is a source of vitamins A and D. Dose amounts should not exceed the recommended dietary allowances (RDAs) of these vitamins. For vitamin A, the RDAs are 900 mcg (3000 units of retinol) daily for men and 700 mcg (2333 units of retinol) daily for women (7135). For vitamin D the RDAs are 15 mcg (600 units) daily for all adults up to 70 years of age, and 20 mcg (800 units) daily for older adults (91703).
POSSIBLY UNSAFE ...when used orally in excessive doses. Avoid doses of cod liver oil that contain more than the Tolerable Upper Intake levels (ULs) of vitamins A and D. For vitamin A the ULs are 3000 mcg (10,000 units of retinol) daily for adults and 2800 mcg (9333 units of retinol) daily for teenagers aged 14 years to 18 years (7135). For vitamin D, the UL is 100 mcg (4000 units) daily for everyone 9 years of age and older (91703). There is also concern that higher doses might increase bleeding risk. Cod liver oil in doses of 20-40 mL daily has been associated with prolonged bleeding time and reduced platelet aggregation in healthy volunteers (4025,4026,10083,91701,91702). There is insufficient reliable information available about the safety of cod liver oil when used topically.
CHILDREN: LIKELY SAFE
when used orally and appropriately in doses providing no more than the recommended dietary allowances (RDAs) of vitamins A and D.
For vitamin A, the RDAs are 400 mcg (1333 units of retinol) daily up to age 6 months, 500 mcg (1667 units of retinol) daily for ages 6 months to 12 months, 300 mcg (1000 units of retinol) daily for ages 1 year to 3 years, 400 mcg (1333 units of retinol) daily for ages 4 years to 8 years, and 600 mcg (2000 units of retinol) daily for ages 9 years to 13 years (7135). For vitamin D, the RDAs are 10 mcg (400 units) daily for infants up to 12 months of age, and 15 mcg (600 units) daily for ages 1 year to 13 years (91703).
In clinical studies, children aged 6 months to 1 year have safely received cod liver oil 2.5 mL daily for 5 months, and children aged 1 year to 5 years have safely received 5 mL daily for 5 months (94756).
CHILDREN: POSSIBLY UNSAFE
when used orally in excessive doses.
Avoid doses of cod liver oil that contain more than the Tolerable Upper Intake levels (ULs) of vitamins A and D. For vitamin A, the ULs are 600 mcg (2000 units of retinol) daily for infants and children up to 3 years of age, 900 mcg (3000 units of retinol) daily for ages 4 years to 8 years, and 1700 mcg (5667 units of retinol) daily for ages 9 years to 13 years (7135). For vitamin D, the ULs are 25 mcg (1000 units) daily for infants up to 6 months, 38 mcg (1500 units) daily for ages 7 months to 12 months, 63 mcg (2500 units) daily for ages 1 year to 3 years, 75 mcg (3000 units) daily for ages 4 years to 8 years, and 100 mcg (4000 units) daily for ages 9 years and above (91703).
PREGNANCY: POSSIBLY SAFE
when used orally and appropriately in doses providing no more than the recommended dietary allowances (RDAs) of vitamins A and D.
For vitamin A, the RDAs are 770 mcg (2567 units of retinol) daily for pregnant women over 18 years of age, and 750 mcg (2500 units of retinol) daily for pregnant teenagers 14 years to 18 years of age (7135). For vitamin D, the RDA is 15 mcg (600 units) daily for pregnant women of all ages (91703).
In clinical research, cod liver oil (Peter Möller, Oslo, Norway) 10 mL orally daily (providing vitamin A 1170 mcg and vitamin D 10 mcg daily) has been used safely from week 17 of pregnancy until delivery, and continuing for 3 months during lactation (94763).
PREGNANCY: POSSIBLY UNSAFE
when used orally in excessive doses.
Avoid doses of cod liver oil which contain more than the Tolerable Upper Intake levels (ULs) of vitamins A and D. For vitamin A, the ULs are 3000 mcg (10,000 units of retinol) daily for pregnant women over 18 years of age, and 2800 mcg (9333 units of retinol) daily for pregnant teenagers aged 14 years to 18 years (7135). For vitamin D, the UL is 100 mcg (4000 units) daily for pregnant women of all ages (91703).
LACTATION: POSSIBLY SAFE
when used orally and appropriately in doses providing no more than the recommended dietary allowances (RDAs) of vitamins A and D.
For vitamin A, the RDAs are 1300 mcg (4333 units of retinol) daily for lactating women over 18 years of age, and 1200 mcg (4000 units of retinol) daily for pregnant teenagers aged 14 years to 18 years (7135). For vitamin D, the RDA is 15 mcg (600 units) daily for lactating women of all ages (91703). An Icelandic cod liver oil, 10 mL daily, has been used safely for 4 months during lactation (94764).
LACTATION: POSSIBLY UNSAFE
when used orally in excessive doses.
Avoid doses of cod liver oil which contain more than the Tolerable Upper Intake levels (ULs) of vitamins A and D. For vitamin A, the ULs are 3000 mcg (10,000 units of retinol) daily for lactating women over 18 years of age, and 2800 mcg (9333 units of retinol) daily for lactating teenagers aged 14 years to 18 years (7135). For vitamin D, the UL is 100 mcg (4000 units) daily for lactating women of all ages (91703).
LIKELY SAFE ...when used orally and appropriately. DHA has been used safely in studies lasting up to 4 years (1016,1043,6413,10321,10869,11333,90684). Fish oil supplements containing DHA have also been safely used in studies lasting up to 7 years (1016). While doses of DHA up to 4 grams orally daily have been used safely in some clinical research (6143), there is some concern that high intake of omega-3 fatty acids such as DHA might increase the risk of bleeding. For this reason, the US Food and Drug Administration (FDA) recommends that consumers limit intake of DHA plus eicosapentaenoic acid (EPA), another omega-3 fatty acid also found in fish oil, to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739).
POSSIBLY SAFE ...when used intravenously and appropriately, in combination with eicosapentaenoic acid (EPA), short-term. Daily infusions with an omega-3 fatty acid-based lipid emulsion (Omegavenous 10%, Fresenius Aktiengesellschaft) providing 4.2 grams/day of DHA and EPA has been used safely for 14 days (1004).
POSSIBLY UNSAFE ...when used orally in high doses. Doses greater than 3 grams daily might decrease platelet aggregation and increase the risk of bleeding (1313). The US Food and Drug Administration (FDA) recommends that consumers limit intake of DHA plus eicosapentaenoic acid (EPA), another omega-3 fatty acid, to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739).
CHILDREN: LIKELY SAFE
when used orally and appropriately.
DHA is a component of some infant formula (424,1045,5708,5941,7599,14403,15003,15495,17735,48088)(48194,48266,48343,90665,90713,90716,110357). In children 7 years and older, DHA 30 mg/kg daily has been used safely for up to 4 years (90684). Also, DHA 0.4-1 grams daily has been safely used in children ages 4 years and older for up to 1 year (11333,90665,100940,104560).
CHILDREN: POSSIBLY UNSAFE
when used orally in preterm infants born less than 29 weeks gestation.
Although not all findings agree (110356,110359), supplementation with an enteral emulsion containing DHA 40 mg/kg to 60 mg/kg daily might increase the risk of developing or worsening bronchopulmonary dysplasia compared to control emulsion (96523,110359).
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally and appropriately.
An intake of DHA 650 mg daily from food and/or supplements during pregnancy seems to be required to prevent a reduction in DHA status before delivery (110329). DHA is commonly used during pregnancy and lactation and is a component of some prenatal supplements. DHA is a normal component of breast milk, with higher levels in breast milk following term vs. preterm pregnancies (14393,14394,14396,14400,14403,14397,20000,47977,47994,48095)(90672,90718,110355). When taken as a prenatal supplement, DHA increases DHA levels in breast milk (90685). Doses of DHA ranging from 300-600 mg daily beginning during the first trimester of pregnancy have been used safely in clinical research (90672,90676,90687,90694). When taken during lactation, DHA increases DHA levels in breast milk (109214,110362). When initiated within 72 hours of delivery of a very preterm infant, taking DHA 1.2 grams daily increases DHA levels in breast milk within 14 days (109214). One study found that DHA supplementation during lactation increased the risk of bronchopulmonary dysplasia in breast-feeding infants born less than 29 weeks gestational age (104559); however, it is unclear if this was due to DHA or various confounding factors. The tolerable upper intake level of DHA during pregnancy or lactation has not been established; most experts recommend DHA 200-300 mg daily. While it is typically advised that this need is met by consuming 8-12 ounces of seafood weekly during pregnancy and 4-8 ounces weekly during lactation, those with nutrient deficiency or those following a vegan diet may meet this need with supplementation (95740,95741).
LIKELY SAFE ...when fish oil or prescription EPA is used orally and appropriately as a source of EPA. Fish oil containing EPA has been used safely for up to 7 years (1016,7819,15497). While doses of prescription EPA (Vascepa, formerly ARM101, Amarin) have been used safely at doses up to 4 grams daily (91409,91410,95715,99136), there is some concern that high intake of omega-3 fatty acids such as EPA might increase the risk of bleeding. For this reason, the US Food and Drug Administration (FDA) recommends that consumers limit intake of EPA plus docosahexaenoic acid (DHA), another omega-3 fatty acid also found in fish oil, to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739).
POSSIBLY SAFE ...when algal oil is used orally and appropriately as a source of EPA. A specific algal oil supplement (Almega PL) providing EPA 250 mg daily has been used with apparent safety for up to 12 weeks (103314). ...when used intravenously under the guidance of a healthcare professional. Fish oil or omega-3 fatty acid lipid emulsions containing EPA, administered intravenously for 1-4 weeks, have been safely used (1004,66042,66421,89323).
POSSIBLY UNSAFE ...when used orally in high doses. Doses greater than 3 grams daily might decrease blood coagulation and increase the risk of bleeding (1313). The US Food and Drug Administration (FDA) recommends that consumers limit intake of EPA plus DHA, another omega-3 fatty acid, to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739).
PREGNANCY AND LACTATION:
Insufficient reliable information available; avoid using.
LIKELY SAFE ...when used orally or intramuscularly and appropriately. Vitamin A, as pre-formed vitamin A (retinol or retinyl ester), is safe in adults when taken in doses below the tolerable upper intake level (UL) of 10,000 IU (3000 mcg) per day (7135). Higher doses increase the risk of side effects. There is also growing concern that taking high doses of antioxidants such as vitamin A might do more harm than good. In an analysis of studies, taking vitamin A supplements alone or in combination with other antioxidants is associated with an increased risk of mortality from all causes (15305,90775). Keep in mind that vitamin A is available in two different forms: pre-formed vitamin A (retinol or retinyl ester) and provitamin A (carotenoids). The safety concerns associated with high vitamin A intake occur with pre-formed vitamin A only. Some supplements contain vitamin A in both pre-formed and provitamin A forms. For these supplements, the amount of pre-formed vitamin A should be used to determine if the amount of vitamin A is safe.
POSSIBLY SAFE ...when used topically and appropriately, short-term. Retinol 0.5% has been used on the skin daily for up to 12 weeks with apparent safety. No serious adverse effects have been reported in clinical trials (103671,103680).
POSSIBLY UNSAFE ...when used orally in high doses. Doses higher than the UL of 10,000 IU (3000 mcg) per day of pre-formed vitamin A (retinol or retinyl ester) might increase the risk of side effects (7135). While vitamin A 25,000 IU (as retinyl palmitate) daily for 6 months followed by 10,000 IU daily for 6 months has been used with apparent safety in one clinical trial (95052), prolonged use of excessive doses of vitamin A can cause significant side effects such as hypervitaminosis A. The risk for developing hypervitaminosis A is related to total cumulative dose of vitamin A rather than a specific daily dose (1467,1469). There is also concern that taking high doses of antioxidants such as vitamin A might do more harm than good. In an analysis of studies, taking vitamin A supplements alone or in combination with other antioxidants is associated with an increased risk of mortality from all causes (15305,90775). There is insufficient reliable information available about the safety of using sublingual formulations of vitamin A.
CHILDREN: LIKELY SAFE
when used orally or intramuscularly and appropriately.
The amount of pre-formed vitamin A (retinol or retinyl ester) that is safe depends on age. For children up to 3 years of age, doses less than 2000 IU (600 mcg) per day seem to be safe. For children ages 4 to 8, doses less than 3000 IU (900 mcg) per day seem to be safe. For children ages 9 to 13, doses less than 5667 IU (1700 mcg) per day seem to be safe. For children 14 to 18, doses less than 9333 IU (2800 mcg) per day seem to be safe (7135). Keep in mind that vitamin A is available in two different forms: pre-formed vitamin A (retinol or retinyl ester) and provitamin A (carotenoids). The safety concerns associated with high vitamin A intake occur with pre-formed vitamin A only. Some supplements contain vitamin A in both pre-formed and provitamin A forms. For these supplements, the amount of pre-formed vitamin A should be used to determine if the amount of vitamin A is safe.
CHILDREN: POSSIBLY UNSAFE
when pre-formed vitamin A (retinol or retinyl ester) is used orally in excessive doses.
For children up to 3 years of age, avoid doses greater than 2000 IU (600 mcg) per day. For children ages 4 to 8, avoid doses greater than 3000 IU (900 mcg) per day. For children ages 9 to 13, avoid doses greater than 5667 IU (1700 mcg) per day. For children ages 14 to 18, avoid doses greater than 9333 IU (2800 mcg) per day (7135). Higher doses of vitamin A supplementation have been associated with increased risk of side effects such as pneumonia, bone pain, and diarrhea (319,95051). Long-term supplementation with low to moderate doses on a regular basis can cause severe, but usually reversible, liver damage (11978).
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally or intramuscularly and appropriately.
Vitamin A, as pre-formed vitamin A (retinol or retinyl ester), is safe during pregnancy and lactation when used in doses less than 10,000 IU (3000 mcg) per day (7135,16823,107293). Keep in mind that vitamin A is available in two different forms: pre-formed vitamin A (retinol or retinyl ester) and provitamin A (carotenoids). The safety concerns associated with high vitamin A intake occur with pre-formed vitamin A only. Some supplements contain vitamin A in both pre-formed and provitamin A forms. For these supplements, the amount of pre-formed vitamin A should be used to determine if the amount of vitamin A is safe.
PREGNANCY AND LACTATION: POSSIBLY UNSAFE
when used orally or intramuscularly in excessive doses.
Daily intake of greater than 10,000 IU (3000 mcg) can cause fetal malformations (3066,7135). Excessive dietary intake of vitamin A has also been associated with teratogenicity (11978). The first trimester of pregnancy seems to be the critical period for susceptibility to vitamin A-associated birth defects such as craniofacial abnormalities and abnormalities of the central nervous system (7135). Pregnant patients should monitor their intake of pre-formed vitamin A (retinol or retinyl ester). This form of vitamin A is found in several foods including animal products, some fortified breakfast cereals, and dietary supplements (3066).
LIKELY SAFE ...when used orally or intramuscularly and appropriately. Vitamin D has been safely used in a wide range of doses (7555,16888,16891,17476,95913,98186,104619,105209,109059). When used orally long-term, doses should not exceed the tolerable upper intake level (UL) of 4000 IU (100 mcg) daily for adults (17506,99773); however, much higher doses such as 50,000 IU (1250 mcg) weekly orally for 6-12 weeks are often needed for the short-term treatment of vitamin D deficiency (16891,17476). Monthly oral doses of up to 60,000 IU (1500 mcg) have also been safely used for up to 5 years (105726). Toxicity usually does not occur until plasma levels exceed 150 ng/mL (17476).
POSSIBLY UNSAFE ...when used orally in excessive doses, long-term. Taking doses greater than the tolerable upper intake level (UL) of 4000 IU (100 mcg) daily for adults for long periods can increase the risk of hypercalcemia (17506); however, much higher doses are often needed for short-term treatment of vitamin D deficiency. Toxicity typically occurs when levels exceed 150 ng/mL (17476).
CHILDREN: LIKELY SAFE
when used orally and appropriately.
When used long-term, doses should not exceed the tolerable upper intake level (UL) of 1000 IU (25 mcg) daily for those 0-6 months of age, 1500 IU (37.5 mcg) daily for those 6-12 months of age, 2500 IU (62.5 mcg) daily for those 1-3 years of age, 3000 IU (75 mcg) daily for those 4-8 years of age, and 4000 IU (100 mcg) daily for those 9 years and older (17506); however, much higher doses are often needed for the short-term treatment of vitamin D deficiency. Some research shows that giving vitamin D 14,000 IU (350 mcg) weekly for a year in children aged 10-17 years is safe (16875). A meta-analysis of clinical studies shows that 1000 IU (25 mcg) daily in those up to a year of age and greater than 2000 IU (50 mcg) daily in those aged 1-6 years does not increase the risk of serious adverse events (108424).
CHILDREN: POSSIBLY UNSAFE
when used orally in excessive doses for longer than one year.
Taking doses greater than the tolerable upper intake level (UL) long-term can increase the risk of hypercalcemia (17506).
PREGNANCY: LIKELY SAFE
when used orally and appropriately.
Vitamin D is safe when used in doses below the tolerable upper intake level (UL) of 4000 IU (100 mcg) daily (17506,95910).
PREGNANCY: POSSIBLY UNSAFE
when used orally in excessive amounts.
Tell patients not to use doses above the tolerable upper intake level (UL) of 4000 IU (100 mcg) daily. Hypercalcemia during pregnancy due to excessive vitamin D intake can lead to several fetal adverse effects, including suppression of parathyroid hormone, hypocalcemia, tetany, seizures, aortic valve stenosis, retinopathy, and mental and/or physical developmental delay (17506).
LACTATION: LIKELY SAFE
when used orally and appropriately.
Vitamin D is safe when used in doses below the tolerable upper intake level (UL) of 4000 IU (100 mcg) daily (17506).
LACTATION: POSSIBLY UNSAFE
when used orally in excessive amounts.
Tell patients not to use doses above the tolerable upper intake level (UL) of 4000 IU (100 mcg) daily (17506).
Below is general information about the interactions of the known ingredients contained in the product Natural Cod Liver Oil. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, cod liver oil may increase the risk of bleeding if used with anticoagulant or antiplatelet drugs.
|
Theoretically, taking cod liver oil with antidiabetes drugs might increase the risk of hypoglycemia.
Some clinical research shows that cod liver oil may lower blood glucose levels in women with gestational diabetes who are already taking antidiabetes drugs when used for at least 12 weeks. However, it does not appear to have any effect on blood glucose when used for shorter durations (101988). Until more is known, use caution.
|
Theoretically, taking cod liver oil with antihypertensive drugs might increase the risk of hypotension.
|
Theoretically, DHA may increase the risk of bleeding if used with anticoagulant or antiplatelet drugs.
Although some clinical evidence suggests that DHA might reduce collagen-stimulated platelet aggregation and thromboxane release, most clinical evidence suggests that DHA alone does not affect blood clotting (11112,11113,48020). However, theoretically, when given in combination with EPA as fish oil, concomitant use with anticoagulant or antiplatelet drugs (including aspirin) might increase risk of bleeding.
|
Theoretically, taking DHA with antidiabetes drugs might reduce the effects of these medications.
In people with type 2 diabetes, including those taking oral hypoglycemic medications, DHA seems to increase fasting blood glucose levels (10321).
|
Theoretically, taking DHA with antihypertensive drugs might increase the risk of hypotension.
|
Theoretically, EPA may increase the risk of bleeding if used with anticoagulant or antiplatelet drugs.
In human research, taking EPA has been shown to inhibit platelet aggregation (9930).
|
Theoretically, taking EPA with antihypertensive drugs might increase the risk of hypotension.
|
Theoretically, taking high doses of vitamin A in combination with other potentially hepatotoxic drugs might increase the risk of liver disease.
|
Concomitant use of retinoids with vitamin A supplements might produce supratherapeutic vitamin A levels.
Retinoids, which are vitamin A derivatives, could have additive toxic effects when taken with vitamin A supplements (3046).
|
Theoretically, taking tetracycline antibiotics with high doses of vitamin A can increase the risk of pseudotumor cerebri.
Benign intracranial hypertension (pseudotumor cerebri) can occur with tetracyclines and with acute or chronic vitamin A toxicity. Case reports suggest that taking tetracyclines and vitamin A concurrently can increase the risk of this condition (10545,10546,10547). Avoid high doses of vitamin A in people taking tetracyclines chronically.
|
Theoretically, high doses of vitamin A could increase the risk of bleeding with warfarin.
Vitamin A toxicity is associated with hemorrhage and hypoprothrombinemia, possibly due to vitamin K antagonism (505). Advise patients taking warfarin to avoid doses of vitamin A above the tolerable upper intake level of 10,000 IU/day for adults.
|
Vitamin D might increase aluminum absorption and toxicity, but this has only been reported in people with renal failure.
The protein that transports calcium across the intestinal wall can also bind and transport aluminum. This protein is stimulated by vitamin D, which may therefore increase aluminum absorption (11595,11597,22916). This mechanism may contribute to increased aluminum levels and toxicity in people with renal failure, when they take vitamin D and aluminum-containing phosphate binders chronically (11529,11596,11597).
|
Vitamin D might reduce absorption of atorvastatin.
A small, low-quality clinical study shows that taking vitamin D reduces levels of atorvastatin and its active metabolites by up to 55%. However, while atorvastatin levels decreased, total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol levels did not substantially change (16828). Atorvastatin is metabolized in the gut by CYP3A4 enzymes, and researchers theorized that vitamin D might induce CYP3A4, causing reduced levels of atorvastatin. However, this proposed mechanism was not specifically studied.
|
Taking calcipotriene with vitamin D increases the risk for hypercalcemia.
Calcipotriene is a vitamin D analog used topically for psoriasis. It can be absorbed in sufficient amounts to cause systemic effects, including hypercalcemia (15). Theoretically, combining calcipotriene with vitamin D supplements might increase the risk of hypercalcemia.
|
Vitamin D might induce CYP3A4 enzymes and reduce the bioavailability of CYP3A4 substrates.
There is some concern that vitamin D might induce CYP3A4. In vitro research suggests that vitamin D induces CYP3A4 transcription. Additionally, observational research has found that increased UV light exposure and serum vitamin D levels are associated with decreased serum levels of CYP3A4 substrates such as tacrolimus and sirolimus, while no association between UV light exposure or vitamin D levels and levels of mycophenolic acid, a non-CYP3A4 substrate, was found (110539). A small, low-quality clinical study shows that taking vitamin D reduces levels of the CYP3A4 substrate atorvastatin and its active metabolites by up to 55%; however, the clinical effects of atorvastatin were not reduced (16828). While researchers theorized that vitamin D might induce CYP3A4, this proposed mechanism was not specifically studied.
|
Theoretically, hypercalcemia induced by high-dose vitamin D can increase the risk of arrhythmia from digoxin.
High doses of vitamin D can cause hypercalcemia. Hypercalcemia increases the risk of fatal cardiac arrhythmias with digoxin (15). Avoid vitamin D doses above the tolerable upper intake level (4000 IU daily for adults) and monitor serum calcium levels in people taking vitamin D and digoxin concurrently.
|
Theoretically, hypercalcemia induced by high-dose vitamin D can reduce the therapeutic effects of diltiazem for arrhythmia.
High doses of vitamin D can cause hypercalcemia. Hypercalcemia can reduce the effectiveness of verapamil in atrial fibrillation (10574). Theoretically this could also occur with diltiazem. Avoid vitamin D doses above the tolerable upper intake level (4000 IU daily for adults) and monitor serum calcium levels in people taking vitamin D and diltiazem concurrently.
|
Theoretically, taking thiazide diuretics and high-dose vitamin D can increase the risk of hypercalcemia.
Thiazide diuretics decrease urinary calcium excretion, which could lead to hypercalcemia if vitamin D supplements are taken concurrently (3072,11541,69580). This has been reported in people being treated with vitamin D for hypoparathyroidism, and also in elderly people with normal parathyroid function who were taking a thiazide, vitamin D, and calcium-containing antacids daily (11539,11540).
|
Hypercalcemia induced by high-dose vitamin D can reduce the therapeutic effects of verapamil for arrhythmia.
Hypercalcemia due to high doses of vitamin D can reduce the effectiveness of verapamil in atrial fibrillation (10574). Avoid vitamin D doses above the tolerable upper intake level (4000 IU daily for adults) and monitor serum calcium levels in people taking vitamin D and verapamil concurrently.
|
Below is general information about the adverse effects of the known ingredients contained in the product Natural Cod Liver Oil. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, cod liver oil is generally well tolerated.
Topically, adverse effects are rare. However, a thorough evaluation of safety outcomes has not been conducted.
Most Common Adverse Effects:
Orally: Flatulence, gastrointestinal upset, heartburn, and nausea.
Serious Adverse Effects (Rare):
Orally: There are rare reports of lipoid pneumonia.
Gastrointestinal ...Orally, flatulence, heartburn, nausea, and gastrointestinal upset are the most frequently reported adverse effects of cod liver oil (3398,94757). Diarrhea also been reported rarely (101990).
Hematologic ...Orally, there is a case report of melena, bleeding duodenal ulcer, and anemia in a 60 year old man who was taking cod liver oil 5 mL daily in combination with other supplements, including fish oil, flaxseed oil, chlorella, and sea buckthorn, which increased his intake of omega-3 fatty acids to more than 21 grams daily (94751).
Immunologic ...Topically, cod liver oil may cause allergic contact dermatitis. There is a case report of allergic contact dermatitis in an 80-year-old woman being treated for leg ulcers with various topical products including 40% cod liver oil ointment (Dermovitamina, Pasquale SRL). She developed severe itchy papules and vesicles requiring treatment with topical corticosteroids and antihistamines, and had a positive skin test to cod liver oil but no other ingredients in the various topical products used (94762).
Neurologic/CNS ...Orally, headache and dizziness have been reported in a single patient each after a single dose of cod liver oil (101990).
Pulmonary/Respiratory
...Data from population surveys of adults in Norway conducted in the 1990s suggests that daily consumption of cod liver oil for at least a month is associated with a 1.
6-fold increase in the odds of adult-onset asthma about 11 years later, when adjusted for several confounding factors (94752). However, the cod liver oil that was commercially available in the 1990s in Norway contained high levels of vitamin A (1000 mcg per 5 mL; equivalent to 3333 units of retinol). It is thought that accumulation of vitamin A in the lungs contributed to the development of asthma.
A case report describes a 63-year-old white woman with a history of smoking and polyarthropathy who underwent a lobectomy after identification of a mass in her lung. This was identified as lipoid pneumonia, thought to be due to her regular intake of cod liver oil capsules (3396).
General
...Orally, DHA is generally well-tolerated when used in doses up to 3 grams daily.
Intravenously, DHA seems to be well tolerated.
Most Common Adverse Effects:
Orally: Belching, fishy aftertaste, loose stools, and nausea.
Serious Adverse Effects (Rare):
Orally: Some case reports raise concerns about increased risk of bleeding with high doses of fish oil containing DHA.
Cardiovascular ...Orally, DHA might increase low-density lipoprotein (LDL) cholesterol levels. However, this appears to be primarily due to increases in the large buoyant type of LDL particles. The small, dense type of LDL particles are reduced (6143,48013,48078,48083,48174,48338).
Dermatologic ...Orally, DHA has been associated with one report of rash and one report of warmth on hands in one clinical study (48217). In another clinical study, two patients taking DHA 400 mg daily reported acne (11333). In another clinical study, one parent of a pediatric patient treated with DHA 600 mg daily reported increased hair loss beginning 6 weeks after completion of supplementation (90699). It is unclear if this adverse effect is specifically related to DHA intake.
Gastrointestinal
...Orally, DHA may cause gastrointestinal upset, fishy aftertaste, belching, flatulence, heartburn, loose stools, anorexia, and dry mouth (10869,11333,48217,109218).
There is also some evidence that increased serum levels of DHA might be associated with an increased risk for atrophic gastritis associated with Helicobacter pylori infection, but further research is needed to clarify this finding (8709).
For fish oils containing EPA and DHA, side effects can include fishy taste, belching, nausea, and loose stools (1009,1313,8699,10007). Three people with pre-existing familial adenomatous polyposis were diagnosed with malignant lesions during the course of long-term fish oil use (999).
Genitourinary ...Orally, one patient in one clinical study who was taking DHA 1, 2, or 4 grams daily (specific dose unclear) reported decreased libido (48217).
Hematologic ...Orally, DHA might cause nose bleeds, but this is uncommon. Onset of severe nose bleeds has been reported in one clinical study in one child who took DHA 600 mg daily (98542). Although most clinical research shows that DHA does not affect blood clotting when taken alone (11112,11113,48020), there is some concern that taking high doses of oils providing DHA along with eicosapentaenoic acid (EPA) might decrease blood coagulation and increase the risk of bleeding (1313). The US Food and Drug Administration (FDA) recommends that consumers limit intake of EPA plus DHA to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739).
Neurologic/CNS ...Orally, DHA may cause dizziness, headache, insomnia, fatigue, and anxiety (10869,11333,48217). In one clinical study, one parent of a pediatric patient treated with DHA 600 mg daily reported increased disruptive behavior in the child (90699).
Ocular/Otic ...Orally, DHA may cause watery eyes but results are inconsistent. In one clinical study, five of 167 infants fed formula containing 0.32% or 0.64% DHA experienced watery eyes. However, none of the infants fed formula containing 0.96% DHA experienced watery eyes (90670). In one clinical study, one patient taking DHA 400 mg daily experienced an ear infection. It is unclear if this event was related to DHA supplementation.
Oncologic ...Orally, DHA may increase the risk of prostate cancer, but additional research is needed to clarify this finding. A meta-analysis of data from observational studies found that higher dietary intake of DHA is associated with a non-linear increased risk of prostate cancer (90677). It is unclear if supplemental DHA intake is associated with increased risk of prostate cancer.
Pulmonary/Respiratory ...Orally, worsened asthma symptoms were reported by one parent of one patient with asthma taking DHA 600 mg daily (90699).
General
...Orally, prescription EPA or EPA derived from fish oil is generally well tolerated in doses of up to 3 grams daily.
Agal oil providing EPA seems to be well tolerated. Doses of EPA greater than 3 grams daily are possibly unsafe.
Intravenously, fish oil or omega-3 fatty acid lipid emulsions containing EPA seem to be well tolerated.
Most Common Adverse Effects:
Orally: Belching, diarrhea, epigastric discomfort, fishy aftertaste, and nausea.
Serious Adverse Effects (Rare):
Orally: Some case reports raise concerns about increased risk of bleeding with high doses.
Cardiovascular ...Orally, taking the prescription ethyl-EPA product (Vascepa, Amarin) 4 grams daily has been linked to a 1% greater risk of atrial fibrillation or atrial flutter that required hospitalization when compared with placebo (101286).
Dermatologic ...Orally, reported side effects of EPA have included skin rash and itching (15497).
Gastrointestinal ...Orally, reported side effects of EPA have included nausea, diarrhea, and epigastric discomfort (15497,103314,110365,110366). For fish oils containing EPA and docosahexaenoic acid, side effects can include fishy taste, belching, nausea, and loose stools (10007).
Hematologic ...Orally, reported side effects of EPA, as well as fish oils containing EPA and docosahexaenoic acid (DHA), have included nosebleed (10007,15497). There is some concern that taking high doses of oils providing EPA along with DHA might decrease blood coagulation and increase the risk of bleeding (1313). To reduce this risk, the US Food and Drug Administration (FDA) recommends that consumers limit intake of EPA plus DHA to 3 grams daily, with no more than 2 grams daily from a dietary supplement (95739). The prescription ethyl-EPA product (Vascepa, Amarin) 4 grams daily has been linked to bleeding in 12% of patients, compared with 10% in the placebo group. Serious bleeding occurred in 3% of the Vascepa group compared to 2% in the placebo group (101286).
Immunologic ...There is preliminary evidence that the EPA in fish oil decreases natural killer (NK) cell activity. Due to this effect, there is concern that increased intake of EPA might have some adverse immunologic effects and possibly increase the risk for viral infections and some cancers (8718).
Musculoskeletal ...Orally, EPA may cause musculoskeletal pain in some patients, although results from clinical research are conflicting. In one clinical study, a higher percentage of patients treated with ethyl-EPA 2 or 4 grams daily experienced joint pain compared to placebo (3.4% and 1.7% vs 0.4%, respectively) (91409). However, in another study, slightly fewer patients taking ethyl-EPA 1.8 grams daily experienced joint, lumbar, or muscle pain compared to placebo (1.6% vs 2.0%, respectively) (15497).
Oncologic ...Three people with pre-existing familial adenomatous polyposis have been diagnosed with malignant lesions during the course of long-term high-docosahexaenoic acid fish oil use (999); however, it is unclear if fish oil, or more specifically EPA, was the cause.
General
...Orally, vitamin A is generally well-tolerated at doses below the tolerable upper intake level (UL).
Serious Adverse Effects (Rare):
Orally: In very high doses, vitamin A can cause pseudotumor cerebri, pain, liver toxicity, coma, and even death.
Dermatologic ...Chronic oral use of large amounts of vitamin A causes symptoms of vitamin A toxicity including dry skin and lips; cracking, scaling, and itchy skin; skin redness and rash; hyperpigmentation; shiny skin, and massive skin peeling (7135,95051). Hypervitaminosis A can cause brittle nails, cheilitis, gingivitis, and hair loss (15,95051). Adverse effects from a single ingestion of a large dose of vitamin A is more common in young children than adults (15). In children, approximately 25,000 IU/kg can cause skin redness and generalized peeling of the skin a few days later and may last for several weeks (15).
Gastrointestinal ...There is some evidence that oral vitamin A supplementation might increase the risk of diarrhea in children. Although vitamin A can prevent diarrhea and reduce mortality in malnourished children, doses as low as 10,000 IU weekly for 40 weeks have been associated with diarrhea in well-nourished children (319). Diarrhea (82326,82389), nausea (7135,100329), abdominal pain (95051), abdominal fullness (100329), and vomiting (7135,82559,95051,95055,109755) have been reported following use of large doses of oral vitamin A. Adverse effects from a single ingestion of a large dose of vitamin A is more common in young children than adults (15). In children, approximately 25,000 IU/kg can cause vomiting and diarrhea (15). Chronic use of large amounts of vitamin A causes symptoms of vitamin A toxicity including anorexia, abdominal discomfort, and nausea and vomiting (7135).
Genitourinary ...Hypervitaminosis A can cause reduced menstrual flow (15). Intravaginally, all-trans retinoic acid can cause vaginal discharge, itching, irritation, and burning (9199).
Hematologic ...Hypervitaminosis A can cause spider angiomas, anemia, leukopenia, leukocytosis, and thrombocytopenia (15,95051).
Hepatic ...Since the liver is the main storage site for vitamin A, hypervitaminosis A can cause hepatotoxicity, with elevated liver enzymes such as alanine aminotransferase (ALT, formerly SGPT) and aspartate aminotransferase (AST, formerly SGOT), as well as fibrosis, cirrhosis, hepatomegaly, portal hypertension, and death (6377,7135,95051).
Musculoskeletal
...Vitamin A can increase the risk for osteoporosis and fractures.
Observational research has found that chronic, high intake of vitamin A 10,000 IU or more per day is associated with an increased risk of osteoporosis and hip fracture in postmenopausal adults, as well as overall risk of fracture in middle-aged males (7712,7713,9190). A meta-analysis of these and other large observational studies shows that high dietary intake of vitamin A or retinol is associated with a 23% to 29% greater risk of hip fracture when compared with low dietary intake (107294). High serum levels of vitamin A as retinol also increase the risk of fracture in males. Males with high serum retinol levels are seven times more likely to fracture a hip than those with lower serum retinol levels (9190). Vitamin A damage to bone can occur subclinically, without signs or symptoms of hypervitaminosis A. Some researchers are concerned that consumption of vitamin A fortified foods such as margarine and low-fat dairy products in addition to vitamin A or multivitamin supplements might cause excessive serum retinol levels. Older people have higher levels of vitamin A and might be at increased risk for vitamin A-induced osteoporosis.
Vitamin A's effects on bone resorption could lead to hypercalcemia (95051).
Hypervitaminosis can cause slow growth, premature epiphyseal closure, painful hyperostosis of the long bones, general joint pain, osteosclerosis, muscle pain, and calcium loss from the bones (15,95051). One child experienced severe bone pain after taking vitamin A 600,000 IU daily for more than 3 months (95051). Vitamin A was discontinued and symptoms lessened over a period of 2 weeks. The patient made a full recovery 2 months later.
Neurologic/CNS
...Orally, adverse effects from a single large dose of vitamin A are more common in young children than adults (15).
Headache, increased cerebrospinal fluid pressure, vertigo, and blurred vision have been reported following an acute oral dose of vitamin A 500,000 IU (7135). In children, approximately 25,000 IU/kg can cause headache, irritability, drowsiness, dizziness, delirium, and coma (15). Chronic use of large amounts of vitamin A causes symptoms of vitamin A toxicity including fatigue, malaise, lethargy, and irritability (7135).
There are reports of bulging of the anterior fontanelle associated with an acute high oral dose of vitamin A in infants (7135,90784,95053,95054). In children, approximately 25,000 IU/kg can cause increased intracranial pressure with bulging fontanelles in infants (15). Also, muscular incoordination has been reported following short-term high doses of vitamin A (7135).
A case of intracranial hypertension involving diffuse headaches and brief loss of vision has been reported secondary to topical use of vitamin A. The patient was using over-the-counter vitamin A preparations twice daily including Avotin 0.05% cream, Retin-A gel 0.01%, and Isotrexin gel containing isotretinoin 0.05% and erythromycin 2%, for treatment of facial acne. Upon exam, the patient was noted to have bilateral optic disc edema. The patient discontinued use of topical vitamin A products. Two months later, the patient reported decreased headaches and an improvement in bilateral optic disc edema was seen (95056).
Ocular/Otic ...In children, oral vitamin A approximately 25,000 IU/kg can cause swelling of the optic disk, bulging eyeballs, and visual disturbances (15). Adverse effects from a single ingestion of a large dose of vitamin A are more common in young children than adults (15).
Oncologic ...There is concern that high intake of vitamin A might increase some forms of cancer. Population research suggests high vitamin A intake might increase the risk of gastric carcinoma (9194).
Psychiatric ...Chronic oral use of large amounts of vitamin A causes symptoms of vitamin A toxicity, which can include symptoms that mimic severe depression or schizophrenic disorder (7135).
Pulmonary/Respiratory ...There is some evidence that oral vitamin A supplementation might increase the risk of pneumonia and diarrhea in children. Although vitamin A can prevent diarrhea and reduce mortality in malnourished children, doses as low as 10,000 IU weekly for 40 weeks have been associated with pneumonia and diarrhea in well-nourished children (319). In preschool children, high-dose vitamin A also increases the risk of respiratory infection (82288).
Other ...Chronic use of large amounts of vitamin A (>25,000 IU daily for more than 6 years or 100,000 IU daily for more than 6 months) can cause symptoms of vitamin A toxicity including mild fever and excessive sweating (7135). High intakes of vitamin A may result in a failure to gain weight normally in children and weight loss in adults (15).
General
...Orally or intramuscularly, vitamin D is well tolerated.
Serious Adverse Effects (Rare):
Orally or intramuscularly: Excessive doses can lead to vitamin D toxicity with symptoms of hypercalcemia, and also sometimes azotemia and anemia.
Cardiovascular ...Vitamin D intoxication can occur when vitamin D supplements are taken orally in excessive doses. Rarely, people develop hypertension (10142). An analysis of clinical research suggests that, when taken orally, vitamin D might modestly increase levels of low-density lipoprotein (LDL)-cholesterol. However, it is not clear if this increase is clinically significant (84642).
Gastrointestinal ...Orally, vitamin D may cause dry mouth. In clinical research, intake of vitamin D 50,000 IU weekly for 4 weeks followed by 50,000 IU monthly for 5 months thereafter was associated with a 3.7-fold increase in reports of dry mouth compared with placebo (91348).Vitamin D intoxication can occur when vitamin D supplements are taken orally in excessive doses. Symptoms of vitamin D toxicity include pancreatitis (10142,84433). Vomiting occurred in one patient given a single dose of 200,000 IU (104624).
Genitourinary ...Vitamin D intoxication can occur when vitamin D supplements are taken orally in excessive doses. Advanced symptoms may include decreased libido (10142). Vaginal discharge and itching have been reported in a clinical trial following oral use (91348).
Hematologic
...Lab values of urinary and blood calcium, phosphate, albumin, blood urea nitrogen, serum cholesterol, aspartate aminotransferase, and alanine aminotransferase concentrations might increase with vitamin D use, especially with high doses (10142,91349,93943).
A case of elevated international normalized ration (INR) has been reported for an 84 year-old patient who took vitamin D 50,000 IU daily for 2 months. The patient's serum levels of vitamin D increased from <7 ng/mL to 100 ng/mL over 6 months. To resolve symptoms, vitamin D supplementation was discontinued (84433).
Musculoskeletal ...Vitamin D intoxication can occur when vitamin D supplements are taken in excessive doses (10142,17506). Symptoms of vitamin D toxicity include osteoporosis in adults and decreased growth in children (10142).
Ocular/Otic ...Vitamin D intoxication can occur when vitamin D supplements are taken orally in excessive doses (10142,17506). Symptoms of vitamin D toxicity include calcific conjunctivitis and photophobia (10142).
Psychiatric ...Vitamin D intoxication can occur when vitamin D supplements are taken orally in excessive doses (10142,17506). In rare cases, symptoms of vitamin D toxicity include psychosis (10142,93002).
Pulmonary/Respiratory ...Vitamin D intoxication can occur when vitamin D supplements are taken orally in excessive doses. Advanced symptoms of vitamin D toxicity may include runny nose (10142,17506,93002).
Renal ...Vitamin D intoxication can occur when vitamin D supplements are taken orally in excessive doses. Symptoms of vitamin D toxicity include azotemia. Vitamin D may also cause hypercalcemia, with advanced symptoms including kidney stones or kidney insufficiency due to precipitation of calcium phosphate in the tubules. Symptoms of renal impairment include frequency, nighttime awakening to urinate, thirst, inability to concentrate urine, and proteinuria. Renal impairment is usually reversible with discontinuation of vitamin D supplements (10142,93002,93943,110831,110833).
