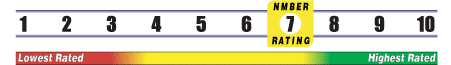
Each capsule contains: L-Lysine 1000 mg. Other Ingredients: Gelatin, Glycerin, Microcrystalline Cellulose, Purified Water.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.

In 2004, Canada began regulating natural medicines as a category of products separate from foods or drugs. These products are officially recognized as "Natural Health Products." These products include vitamins, minerals, herbal preparations, homeopathic products, probiotics, fatty acids, amino acids, and other naturally derived supplements.
In order to be marketed in Canada, natural health products must be licensed. In order to be licensed in Canada, manufacturers must submit applications to Health Canada including information about uses, formulation, dosing, safety, and efficacy.
Products can be licensed based on several criteria. Some products are licensed based on historical or traditional uses. For example, if an herbal product has a history of traditional use, then that product may be acceptable for licensure. In this case, no reliable scientific evidence is required for approval.
For products with non-traditional uses, some level of scientific evidence may be required to support claimed uses. However, a high level of evidence is not necessarily required. Acceptable sources of evidence include at least one well-designed, randomized, controlled trial; well-designed, non-randomized trials; cohort and case control studies; or expert opinion reports.
Finished products licensed by Health Canada must be manufactured according to Good Manufacturing Practices (GMPs) as outlined by Health Canada.
Below is general information about the effectiveness of the known ingredients contained in the product L Lysine HCl 1000 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product L Lysine HCl 1000 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
POSSIBLY SAFE ...when used orally in doses up to 3000 mg daily for up to one year (1114,1119,1120,90642,104104), or up to 6000 mg daily for up to 8 weeks (90644,90645). ...when used topically and appropriately, short-term (11051).
PREGNANCY AND LACTATION:
Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product L Lysine HCl 1000 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, lysine may reduce the effects of 5-HT4 agonists.
Animal research suggests that L-lysine is a partial serotonin receptor 4 (5-HT4) antagonist and inhibits diarrhea induced by the 5-HT4 agonist, 5-hydroxytryptophane (19400).
|
Below is general information about the adverse effects of the known ingredients contained in the product L Lysine HCl 1000 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally and topically, lysine is generally well tolerated.
Most Common Adverse Effects:
Orally: Abdominal pain, diarrhea, and dyspepsia.
Gastrointestinal ...Orally, lysine has been reported to cause diarrhea and abdominal pain, including dyspepsia (1114,1115,1116,1118,1120).
Renal ...There is one case report of oral lysine use associated with tubulointerstitial nephritis progressing to chronic renal failure in a 44-year old female (1121).
