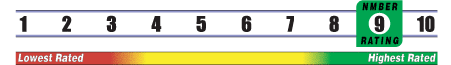
Each capsule contains: Phytosterols 500 mg, providing Beta-Sitosterol, Campesterol and Sigmasterol combined free Phytosterols 450 mg. Other Ingredients: Cellulose, Hypromellose, Magnesium Stearate.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.

In 2004, Canada began regulating natural medicines as a category of products separate from foods or drugs. These products are officially recognized as "Natural Health Products." These products include vitamins, minerals, herbal preparations, homeopathic products, probiotics, fatty acids, amino acids, and other naturally derived supplements.
In order to be marketed in Canada, natural health products must be licensed. In order to be licensed in Canada, manufacturers must submit applications to Health Canada including information about uses, formulation, dosing, safety, and efficacy.
Products can be licensed based on several criteria. Some products are licensed based on historical or traditional uses. For example, if an herbal product has a history of traditional use, then that product may be acceptable for licensure. In this case, no reliable scientific evidence is required for approval.
For products with non-traditional uses, some level of scientific evidence may be required to support claimed uses. However, a high level of evidence is not necessarily required. Acceptable sources of evidence include at least one well-designed, randomized, controlled trial; well-designed, non-randomized trials; cohort and case control studies; or expert opinion reports.
Finished products licensed by Health Canada must be manufactured according to Good Manufacturing Practices (GMPs) as outlined by Health Canada.
Below is general information about the effectiveness of the known ingredients contained in the product Bps Phytosterols. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Bps Phytosterols. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Plant sterols have been safely used in studies lasting up to one year (35022,35037,90724,90726,90730,90732,90733,90734,90739,90742). Due to the unknown safety of plant sterol oxidation products, and the known production of these oxidation products during the heating of foods (106092), there is insufficient reliable information available about the safety of heat-treated foods containing added plant sterols.
CHILDREN: LIKELY SAFE
when used orally and appropriately.
Plant sterols have been safely used in children in studies lasting for up to 6 months (35037,90722,90723,90726,90731,35106).
PREGNANCY AND LACTATION: Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product Bps Phytosterols. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Below is general information about the adverse effects of the known ingredients contained in the product Bps Phytosterols. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General ...Orally, plant sterols are generally well tolerated.
Dermatologic ...Acute dermatitis has been reported in a case report of a 51-year-old female who used a pharmacy-brand plant sterol supplement to lower cholesterol levels. The rash was widespread with lesions on the hands and feet and a red and swollen face. The patient was treated orally with prednisolone and topically with emollients for 2 weeks (90737). It is not entirely clear if this adverse effect was due to the plant sterol supplement or another contributing factor.
Endocrine ...Peripheral precocious puberty has been described in a 20-month-old boy who ingested about 15 grams daily of a chicken essence product containing the plant sterol avenasterol, also known as clerosterol or chondrillasterol. Signs and symptoms included elevated estradiol and testosterone levels, acne, hairiness, increased penis size, hoarse voice, and increased growth rate. These effects were reversed when the product was stopped (112084).
Gastrointestinal ...Abdominal discomfort has occurred in one child in a clinical trial of oral plant sterols (35106). Because plant sterols decrease cholesterol absorption in the gut, there is concern that they might produce some gastrointestinal symptoms like diarrhea and excess amounts of fat in the stool (steatorrhea), although these adverse effects have not been reported in clinical research.
