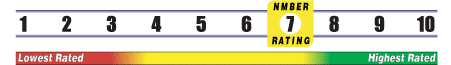
Each capsule contains: Dehydroepiandrosterone (dehydroepiandrosterone, time-released) 50 mg. Other Ingredients: Kosher Gelatin (capsule), Cellulose, Magnesium Stearate.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.
Below is general information about the effectiveness of the known ingredients contained in the product DHEA 50 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product DHEA 50 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
POSSIBLY SAFE ...when used orally and appropriately, short-term. Most studies have been small and lasted from a few weeks to 6 months, with usual doses of 50 mg daily (793,1635,2133,3231,4249,4251,4252,4253,4254,4255,9691)(9692,10986,12215,12564,14277,21416,88726,90304,99925). Some studies have also used oral DHEA with apparent safety for 12-24 months (2113,6446,10406,11464,12561,15027,88492). ...when used intravaginally and appropriately. Intravaginal ovules of DHEA 3.25 mg to 13 mg have been safely used for up to 12 weeks (21320,21429,21430). ...when used topically and appropriately. A DHEA cream 1% to 10% has been safely used for up to 12 months (4242,21428).
POSSIBLY UNSAFE ...when used orally in high doses or long-term. There is concern that long-term use or use of amounts that cause higher than normal physiological DHEA levels might increase the risk of prostate cancer (2111,12565), breast cancer (10370,10401,10403), or other hormone-sensitive cancers (6445). In some cases, 50-100 mg daily can produce slightly higher than normal physiological DHEA levels (4249,4251). There is insufficient reliable information available about the safety of using DHEA intravenously or intramuscularly.
PREGNANCY AND LACTATION: POSSIBLY UNSAFE
when used orally.
DHEA can cause higher than normal androgen levels (2133,4249,4251,4253), which might adversely affect pregnancy or a nursing infant.
Below is general information about the interactions of the known ingredients contained in the product DHEA 50 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, DHEA might increase the risk of bleeding if used with anticoagulant or antiplatelet drugs.
|
Theoretically, DHEA might increase the risk of psychiatric adverse events when used with antidepressants.
In a human case report, the use of a selective serotonin reuptake inhibitor (SSRI) with DHEA caused a manic episode (7023). Concern for this interaction may be greater in younger individuals with higher baseline DHEA levels.
|
Theoretically, DHEA might interfere with the clinical effects of aromatase inhibitors.
DHEA is a potent estrogen agonist, which may antagonize the anti-estrogen activity of aromatase inhibitors (10401).
|
Theoretically, DHEA might increase the levels of drugs metabolized by CYP3A4.
Some preliminary evidence shows that DHEA may inhibit CYP3A4 (1389); however, the clinical significance of this potential interaction is not known.
|
Theoretically, DHEA might increase the effects and adverse effects of estrogen therapy.
DHEA is a precursor to estrogen and androgen and is metabolized into those substances. In clinical research, DHEA supplements increase the levels of these hormones (6012,7614,8593,10986,12651,12564,15027,21321,21323,21324)(21325,21326,21327,21328,21330,21331,21356,21364,21389,21393)(21397,21398,21417,21419,21427,47273,47348,88375,90304). Also, in clinical research, estrogen-progestin oral contraceptives and conjugated estrogens reduce blood levels of DHEA and DHEA-S (21372,21373,21374,21437,21438). The clinical significance of these findings is unclear.
|
Theoretically, DHEA might interfere with the anti-estrogen effects of fulvestrant.
|
Theoretically, DHEA might interfere with the anti-estrogen effects of tamoxifen.
|
Theoretically, DHEA might increase the effects and side effects of testosterone therapy.
DHEA is a precursor to estrogen and androgen and is metabolized into those substances. In clinical research, DHEA supplements increase the levels of these hormones (6012,7614,8593,10986,12651,12564,15027,21321,21323,21324)(21325,21326,21327,21328,21330,21331,21356,21364,21389,21393)(21397,21398,21417,21419,21427,47273,47348,88375,90304,99924,99925,104162). The clinical significance of these findings is unclear.
|
DHEA can increase blood levels of triazolam.
Administration of DHEA 200 mg daily for two weeks was shown to inhibit the cytochrome P450 3A4 (CYP3A4) metabolism of triazolam. This inhibition appears to be due to DHEA-S, rather than DHEA (1389).
|
DHEA might reduce the effectiveness of the tuberculosis vaccine.
Animal research shows that high doses of DHEA can reduce the efficacy of the Bacillus Calmette-Guérin (BCG) tuberculosis vaccine (21316).
|
Below is general information about the adverse effects of the known ingredients contained in the product DHEA 50 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally and topically, DHEA seems to be well tolerated when used in typical doses, short-term.
However, there is some concern that long-term oral use of DHEA may be linked to a greater risk for cancer.
Most Common Adverse Effects:
Orally: Acne, headache, insomnia, mood changes, and nausea. In females, masculinization symptoms including deepening of the voice, increased size of genitals, irregular menses, oily skin, reduced breast size, and unnatural hair growth. In males, aggression, breast tenderness or enlargement (gynecomastia), urinary urgency, and testicular wasting.
Serious Adverse Effects (Rare):
Orally: Possible increased risk for cardiovascular events and various types of cancer.
Cardiovascular ...Incidences of arrhythmia (21334,47540), chest pain (21332,21333), palpitations (21332,21333,88492), hypertension, and transient ischemic attacks (21353,21354,47300) have been reported. DHEA has also been found to decrease high-density lipoprotein (HDL) levels (21344,21345,21346,21347,21348,21349) and increase triglycerides (21334).
Dermatologic ...Acne has been the most commonly reported adverse effect in human research, particularly in females (2113,2114,4242,7614,7559,12561,12574,21346,21351,21354)(21355,21356,21357,21358,21360,21361,21362,21363,21364,47300)(47355,47409,90304,103185). However, it is generally mild and may be treated by reducing the dose (7559). Incidences of contact dermatitis (47402), acneiform dermatitis (2113), greasy hair and skin (17218,21351,21355,21363,21387,21389,47355), keratosis (47402), skin rash (12574,21361,21363), erythema (21334), scalp itching (17218,21357), and skin spots (21387) have also been reported. Increased hair growth and hirsutism have been noted in several clinical trials, including the development of mild mustache in females (2114,4242,12561,12574,17218,21346,21351,21354,21355,21358) (21361,21362,21363,21370,21387,21389,21415,47300). Increased perspiration and odor have also been reported in human research (17218,21354,21356,21357).
Endocrine ...In postmenopausal patients, high doses of DHEA (1600 mg daily) induced insulin resistance, reportedly due to increased androgen levels that occurred during supplementation (21324).
Gastrointestinal ...Gastrointestinal disturbances such as nausea, diarrhea, and abdominal discomfort have been noted in human research (2111,6098,7559,12574,21348,21358,21386).
Genitourinary ...In older adults, elevated and severe urinary symptoms (as evidenced by scores of more than 20, using the American Urological Association Symptom Index for Benign Prostatic Hyperplasia [International Prostate Symptom Score]) and urinary tract infection were reported (21353). Rare incidences of abnormal menses (2114) and increased discharge (21415) have been reported. DHEA has been associated with hematuria (47300).
Hepatic ...Elevated liver enzymes have been reported following DHEA supplementation (21364,47300). However, an analysis of multiple studies in varied patient populations taking DHEA supplements found no elevations in liver enzymes (107791).
Musculoskeletal ...Incidences of asthenia, arthralgia, and myalgia, including calf cramps, have been reported (12574,21354,21358,21365,47355).
Neurologic/CNS ...In humans, dizziness, fatigue, malaise, sleep disturbances, increased dreaming, night sweats, restlessness, "painful spots," and a crawling scalp sensation have been reported (3865,21354,21363,21389). There is a case of seizure associated with DHEA use in a 30 year-old female with fragile X syndrome and no history of convulsive disorder who used DHEA to try to improve ovarian production (47344).
Ocular/Otic ...In patients with Sjögren syndrome, maculae lesions, ocular pain and dryness, and painful eye exams have been reported (21358,21363,21365).
Oncologic ...Preclinical research suggests that DHEA may increase the risk of cancer, particularly prostate, liver, breast, and pancreatic cancers (2111,10370,10401,10403,12565,21332,21333,21334,47251,47256)(47366,47388,47539). High concentrations of DHEA in postmenopausal patients have been associated with an increased risk of breast cancer (2115,6445).
Psychiatric ...DHEA-induced mania has been reported (5870,6102,7023,21383). Clinical studies have also reported anxiety, nervousness, irritability, emotional change, and depression in patients receiving DHEA (2114,21358,21360,21370).
Pulmonary/Respiratory ...Increased cough and nasal congestion have been noted in human research (3865,11334). A report of acute respiratory failure was made in clinical study evaluating the use of DHEA in patients with myotonic dystrophy (type 1) (21334).
Other ...Perceived increases in weight gain have been reported with use of DHEA (2114,21361).
