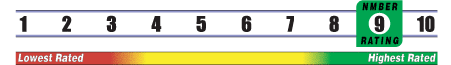
Each 1 tsp serving contains: Nickel 72.6 mcg. Other Ingredients: Glycerol, Potassium Sorbate, Purified Water.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.

In 2004, Canada began regulating natural medicines as a category of products separate from foods or drugs. These products are officially recognized as "Natural Health Products." These products include vitamins, minerals, herbal preparations, homeopathic products, probiotics, fatty acids, amino acids, and other naturally derived supplements.
In order to be marketed in Canada, natural health products must be licensed. In order to be licensed in Canada, manufacturers must submit applications to Health Canada including information about uses, formulation, dosing, safety, and efficacy.
Products can be licensed based on several criteria. Some products are licensed based on historical or traditional uses. For example, if an herbal product has a history of traditional use, then that product may be acceptable for licensure. In this case, no reliable scientific evidence is required for approval.
For products with non-traditional uses, some level of scientific evidence may be required to support claimed uses. However, a high level of evidence is not necessarily required. Acceptable sources of evidence include at least one well-designed, randomized, controlled trial; well-designed, non-randomized trials; cohort and case control studies; or expert opinion reports.
Finished products licensed by Health Canada must be manufactured according to Good Manufacturing Practices (GMPs) as outlined by Health Canada.
Below is general information about the effectiveness of the known ingredients contained in the product Nickel Oligofluide. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Nickel Oligofluide. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Nickel is safe for most adults when used in doses less than the tolerable upper intake level (UL) of 1 mg daily (7135).
POSSIBLY UNSAFE ...when used in high doses and for prolonged periods. Taking nickel in doses above the tolerable upper intake level (UL) of 1 mg daily increases the chance of adverse effects (7135). Taking high doses of nickel is associated with acute toxicity. Prolonged industrial exposure to nickel is also associated with hypersensitivity disorders, lung disorders, and cancer (7135,16307,16311).
CHILDREN: LIKELY SAFE
when used orally and appropriately.
Nickel is safe in children when used in daily doses less than the tolerable upper intake level (UL) of 0.2 mg daily in children 1-3 years, 0.3 mg daily in children 4-8 years, and 0.6 mg daily in children 9-13 years (7135).
CHILDREN: POSSIBLY UNSAFE
when used orally in high doses.
Taking nickel in doses above the UL might not be safe (7135).
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally and appropriately.
Nickel is safe to use during pregnancy and lactation in doses below the tolerable upper intake level (UL) of 1 mg daily (7135). There is insufficient reliable information available about the safety of nickel when taken in doses above the UL during pregnancy and lactation.
Below is general information about the interactions of the known ingredients contained in the product Nickel Oligofluide. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Disulfiram can reduce the absorption and clinical effects of nickel.
Disulfiram can chelate nickel, preventing its absorption. This interaction has been used therapeutically to reduce eczema in people with severe nickel hypersensitivity (16322).
|
Below is general information about the adverse effects of the known ingredients contained in the product Nickel Oligofluide. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, nickel is well tolerated when taken in amounts found in foods, which are typically below the tolerable upper intake level (UL).
However, doses above the UL can cause serious adverse effects.
Most Common Adverse Effects:
All routes of administration: Contact dermatitis.
Serious Adverse Effects (Rare):
Orally: Chronic consumption of large quantities of nickel might increase the risk of cancers of the lung, nose, larynx, and prostate.
Cardiovascular ...Particulate matter-associated nickel exposures have been associated with cardiopulmonary diseases (65021). Nickel exposure may also cause variable degrees of cardiovascular system poisoning (16333). In one case report, a 45-year-old male developed leukocytosis, low-grade fevers, dyspnea, and fatigue after implantation of a cardioverter-defibrillator (ICD). The patient eventually developed fulminant hypereosinophilia and eosinophilic myocarditis, resulting in cardiogenic shock and multiorgan failure. After the patient had been medically stabilized, the ICD was removed, leading to symptom resolution (99670).
Dermatologic
...Orally and topically, nickel can cause dermatitis and other hypersensitivity reactions in sensitized individuals (65045).
Nickel exposure may cause systemic contact dermatitis (65017). Orally, nickel sulfate has been associated with classic systemic allergic contact eczema (64916). Topical exposure can occur from the use of common equipment containing nickel. There are at least four case reports of allergic contact dermatitis of the hand due to the use of a nickel-containing electronic cigarette device. Patients with nickel allergy should be advised to avoid devices that contain nickel (99665,99669). There is also a case report of a 21-year-old female who developed recurrent rash on her upper chest and thighs due to exposure to nickel via weightlifting equipment at her gym (99671).
"Nickel itch" may occur up to seven days before skin eruption occurs. The primary skin eruption is follicular, which may be followed by skin ulceration. Nickel may cause Baboon syndrome, characterized by a clinical presentation of systemic contact dermatitis with a pruritic and confluent maculopapular, light red eruption, localized in the gluteal area and the major flexures and developing several hours or days after nickel contact (64963,65092).
Individuals with mutations in the filaggrin gene may be especially susceptible to hand eczema caused by additive effects from irritants and nickel exposure (65012). Nickel exposure may lead to cross-sensitivity dermatitis reaction in individuals who are allergic to other metals, especially palladium, cobalt, and aluminum (65023,64939,65099,65028,64968,65026).
Gastrointestinal ...Orally, consuming high doses of nickel 0. 5-2.5 grams has been reported to cause nausea, vomiting, abdominal pain, and diarrhea. Patients with systemic nickel allergy syndrome (SNAS) can experience colic and diarrhea after consumption of nickel-rich foods (99663).
Genitourinary ...Nickel has been shown to cross the placenta and to have embryotoxic and teratogenic properties (65068,65091). Nickel may upset the hormonal balance of the mother and may impair development of the preimplantation embryo (65061).
Hepatic ...Orally, nickel has been reported to cause hepatotoxicity (65009).
Immunologic
...Orally and topically, nickel can cause hypersensitivity reactions in sensitized individuals (64916,65017,65045).
Nickel sensitivity, which can cause dermatologic and gastrointestinal symptoms, has an estimated prevalence of 11% in the general population, with a higher overall incidence in females than in males (99668). About 10% of nickel-sensitive people react to ingestion of 550-1300 mcg. Nickel sensitivity, once acquired, may persist indefinitely (64994). Some clinical research suggests that daily consumption of nickel 30 ng, 0.3 mcg, or 1.5 mcg for one year can hyposensitize individuals and reduce symptoms of nickel sensitivity (99663).
In sensitive individuals, dermal or systemic exposure to nickel can induce a type I immediate (anaphylactic) reaction and a type IV delayed hypersensitivity reaction, mediated by secreted cytokines and allergen-specific T lymphocytes (64989,65002). Nickel exposure may cause a cross-sensitivity reaction in individuals who are allergic to other metals, especially palladium, cobalt, and aluminum (65023,64939,65099,65028,64968,65026).
Implantation of devices containing nickel have been reported to cause localized and systemic reactions. A 59-year-old male with a hip arthroplasty, who later tested positive for nickel allergy, experienced hip pain, aseptic implant loosening, and contact allergic dermatitis in the inguinal regions, necessitating multiple surgeries (99664). In another case, a 45-year-old male developed leukocytosis, low-grade fevers, dyspnea, fatigue, and edema immediately and during the two months after implantation of a cardioverter-defibrillator (ICD). The patient eventually developed fulminant hypereosinophilia and eosinophilic myocarditis, resulting in cardiogenic shock and multiorgan failure. Once the patient was stabilized, the ICD was removed and all symptoms resolved. The patient tested positive for nickel allergy (99670).
There have been at least two case reports of hypersensitivity reactions to the nickel contained in implanted cardiac rings. One 57-year-old male experienced severe urticaria and angioedema two weeks after implantation of a mitral valve annuloplasty ring. The patient tested positive for nickel allergy and, although there was no evidence of nickel in his blood, symptoms resolved upon removal of the nickel-containing ring (99667). A 56-year-old male experienced persistent urticarial rash and multiple episodes of anaphylactic shock within the first month after implantation of a mitral ring. The patient tested positive to nickel upon skin prick testing; symptoms resolved after removal of the mitral ring (99666).
Neurologic/CNS ...Orally, consuming large quantities of nickel can lead to acute nickel toxicity, which may cause cerebral edema (16334).
Oncologic
...The International Agency for Research on Cancer has classified nickel compounds as carcinogenic to humans (65046).
Occupational exposure to nickel and nickel compounds in nickel refining, cutlery factories, and alkaline battery manufacturing plants is associated with an increased incidence of lung, bronchial, and nasal cancers (39918,65029,16334,65056,64962,65075,64912,65006,64974). It is unclear if one specific lung cancer cell type is associated with nickel exposure (65054). Nickel fumes present in nickel and some steel refineries are associated with undifferentiated and squamous histologies of the nose and paranasal sinuses (65040,65050,65101,64898,65093,64909,64984,65031,64969). Occupational changes in industrial processes that have eliminated or reduced exposures to nickel compounds have reduced the risk of lung cancer (64966); however, experts believe that further preventive measures are needed to decrease or eliminate occupational nickel exposure time (65010). Exposure to nickel concentrate or metallic nickel through the metallurgical refining process has not been associated with an increase in respiratory cancer risk (64918).
Although clinical and epidemiologic research show that sparingly soluble nickel compounds, and possibly also soluble nickel compounds, may be linked to an increased risk for lung and nasal cancers, there is conflicting evidence as to whether inhalation exposure to soluble nickel alone is carcinogenic (42782,64940). According to some researchers, these opposing positions may be resolved by the hypothesis that high concentrations of inhaled nickel may induce chronic lung inflammation, enhancing carcinogenic risks associated with inhalation exposure to other substances (64938). Therefore, it has been suggested that if exposures are kept below levels that cause chronic respiratory toxicity, carcinogenic effects may be avoided.
There is concern that exposure to nickel in conjunction with another metal might increase the risk for certain forms of cancer. In the art glass industry, an association was found between an increased risk for stomach cancer and the use of a mixture of arsenic, copper, nickel, and manganese (65086). Based on epidemiological data and genotoxicity testing, the combination of nickel and chromium in the fumes from the welding of stainless and high alloy steels may contribute to an additive risk of respiratory tract cancer (65064). Additionally, co-exposure to nickel and cadmium may increase the risk of lung cancer (64945).
Epidemiological studies that have found an association between nickel and an increased risk for cancer might be confounded by the presence of cigarette smoking and asbestos exposure (65087,65106). Nickel occurs in mainstream cigarette smoke in the range of 0-0.51 mg per cigarette (65096). The high prevalence of prostate cancer in smokers may be related to the nickel in cigarettes (65109).
Implantation of nickel-containing hardware has also been reported to induce the development of cancer (64913,65032,65039). In one case, a 65-year-old female developed osteogenic sarcoma at the site of a nail that had been implanted for nine years for fixation of a femoral neck fracture. A concentration of 14 ppm of nickel was detected within the tumor tissue, indicating neoplastic induction by nickel (65032). In a different case, a malignant fibrous histiocytoma of the bone developed 20 years after a femoral fracture treated by plate-screw fixation. Nickel was present in the tumor tissue, indicating the metabolism of corrosion products (64913).
Pulmonary/Respiratory
...Inhalation of nickel carbonyl has been reported to cause nickel toxicity, including pulmonary edema, acute tracheobronchitis, or diffuse interstitial pneumonitis (16334,64905,61495).
Occupational exposure to nickel has been reported to cause chronic rhinitis, sinusitis, airway narrowing, asthma, bronchitis, reduced lung function, lung fibrosis, nasal polyposis, perforation of the nasal septum, and cancer of the lungs and nasal sinuses (65045,64933,65077). Bronchiolitis induced by nickel and nitric acid have also been reported (64948).
Renal ...Orally, nickel has been reported to cause nephrotoxicity (16333,65009), especially when consumed in excessive amounts (65104).
Other
...Orally, nickel toxicity can occur after the consumption of large quantities of nickel.
The level of toxicity caused by nickel exposure seems to depend on its chemical form. Insoluble nickel compounds, including metallic nickel, nickel sulfides, and nickel oxides, seem to have a higher carcinogenic potential than water-soluble nickel salts (chloride, nitrate, sulfate) (64902). Conversely, it has been reported that nickel compounds, other than nickel carbonyl, are essentially nontoxic after ingestion due to their low absorption from the gastrointestinal tract (65052).
The treatment of nickel toxicity is not well studied. In some reports, antioxidants, such as ascorbic acid, may protect against nickel toxicity (65009,64991). Chelating drugs have been used as an antidote in nickel poisoning (65045,64979). Sodium diethyldithiocarbamate and disulfiram have also been proposed as effective nickel chelators in the treatment of acute nickel carbonyl poisoning; however, adequate clinical studies are not available to assess the effectiveness of these options (65003).
