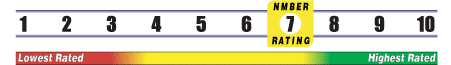
Each capsule contains: Lithium (orotate) 5 mg. Other Ingredients: Microcrystalline Cellulose, Gelatin.
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.
Below is general information about the effectiveness of the known ingredients contained in the product Lith Oro 5 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Lith Oro 5 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when lithium carbonate or lithium citrate is used orally and appropriately. Lithium carbonate and lithium citrate are FDA-approved drugs and have been used safely in clinical studies (15,97770). Lithium has a narrow therapeutic window and plasma levels must be monitored to avoid toxicity (15). Lithium levels should be drawn 12 hours after the last dose of lithium after steady state concentrations have been attained (approximately 3 days). Toxicity is most common at levels of 1.5 mEq/L, although some patients develop toxicity at levels less than 1 mEq/L (15,97770). There is insufficient reliable information available about the safety of lithium aspartate, lithium orotate, or other forms of supplemental lithium.
CHILDREN: POSSIBLY SAFE
when prescription lithium carbonate or lithium citrate is used orally and appropriately under medical supervision in children 7 years of age and older (15).
There is insufficient reliable information available about the safety of lithium aspartate, lithium orotate, or other forms of supplemental lithium.
PREGNANCY: POSSIBLY UNSAFE
when lithium carbonate and lithium citrate are used orally (15).
Lithium can cause fetal toxicity and increases the risk for cardiac and other abnormalities, including neural tube and urethral defects. However, it does not seem to increase the risk for preterm birth or low birth rate (15,9166,97770,104266). Some research suggests lithium might increase the risk for spontaneous abortion. Based on a meta-analysis of 2 population studies, taking lithium during pregnancy may increase the risk for spontaneous abortion when compared with the general population, but not when compared with patients with affective disorders not taking lithium during pregnancy (104266). This suggests that it may be the presence of affective disorder itself, or the possible associated use of other teratogenic drugs or substances during pregnancy, which may increase the risk for spontaneous abortion.
When the potential benefits to the mother and child outweigh the possible risk to the fetus, prescription lithium may be used with close monitoring by a healthcare professional (15,9166,97770,104266). The safety of lithium supplements during pregnancy is unknown.
LACTATION: LIKELY UNSAFE
when used orally.
Lithium is secreted into breast milk and may cause adverse effects in the nursing infant (15). Prescription lithium may be used in circumstances when the potential maternal benefits outweigh the possible risk to the infant. The infant should be closely monitored for signs of lithium toxicity (97770).
Below is general information about the interactions of the known ingredients contained in the product Lith Oro 5 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, taking lithium supplements with ACEIs might increase levels and adverse effects of lithium.
|
Theoretically, taking lithium supplements with anticonvulsants might increase the risk of neurotoxicity.
|
Theoretically, taking lithium supplements with antipsychotic drugs might increase the risk of encephalopathic syndrome.
Encephalopathic syndrome has been reported in multiple patients taking prescription lithium and antipsychotics concomitantly. Symptoms have included weakness and lethargy, fever, confusion, and extrapyramidal symptoms. In some patients, resulting brain damage was irreversible. Although there is no established causal relationship between these symptoms and the combination of lithium and antipsychotic medications, there is a theoretical relationship (97770). It is unclear if this interaction would occur with the smaller doses found in lithium supplements.
|
Theoretically, taking lithium supplements with calcium channel blockers might reduce lithium levels and might also increase the risk of certain adverse effects.
Calcium channel blockers might reduce lithium concentrations. Monitor lithium levels with concurrent use. Calcium channel blockers might also increase the adverse neurological and gastrointestinal adverse effects of lithium (9,15). It is unclear if these interactions would occur with the smaller doses found in lithium supplements.
|
Theoretically, taking lithium supplements with loop diuretics might increase lithium levels and adverse effects.
Thiazide diuretics and loop diuretics might reduce lithium excretion, particularly in sodium-restricted patients (9,15). If lithium is clinically indicated and other treatment options are unavailable or inadequate in patients using diuretics, lithium treatment can be initiated with extreme caution. Serum lithium should be measured frequently and the doses used should be the lowest dose ordinarily tolerated (97770). It is unclear if this interaction would be clinically significant with the smaller doses found in lithium supplements.
|
Theoretically, taking lithium supplements with methyldopa might increase the risk of lithium toxicity.
Concurrent use of methyldopa with lithium increases the risk of lithium toxicity (9). It is unclear if this interaction would be clinically significant with the smaller doses found in lithium supplements.
|
Theoretically, taking lithium supplements with methylxanthines might decrease lithium levels.
|
Theoretically, taking lithium supplements with NSAIDs might increase lithium levels and adverse effects.
|
Theoretically, taking lithium supplements with phenothiazines might decrease the levels and clinical effects of phenothiazines.
|
Theoretically, taking lithium supplements with serotonergic drugs might both mask and increase the risk of serotonin syndrome.
In a case report, a 67-year-old female with depression and bipolar disorder using lithium in combination with selective serotonin reuptake inhibitors (SSRIs) and other medications developed serotonin syndrome with symptoms of deep tendon hyperreflexia, muscle rigidity, tremor, and hyperthermia. However, agitation, one classical symptom of serotonin syndrome, was lacking. This was thought to be due to masking by lithium toxicity (105343). Lithium can increase serotonin levels (9,15), thus, combining serotonergic drugs with lithium might increase the risk of serotonergic side effects including serotonin syndrome and cerebral vasoconstrictive disorders. It is unclear if this interaction would occur with the smaller doses found in lithium supplements.
|
Theoretically, taking lithium supplements with skeletal muscle relaxants might prolong neuromuscular blockade.
|
Below is general information about the adverse effects of the known ingredients contained in the product Lith Oro 5 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, prescription forms of lithium are generally well tolerated when used as prescribed.
Plasma levels must be monitored to avoid toxicity. It is unclear how the lower doses of lithium found in supplements may alter the occurrence and likelihood of these adverse effects.
Most Common Adverse Effects:
Orally: Edema, fatigue, fine tremor, gastrointestinal symptoms, lethargy, muscle weakness, polydipsia, polyuria, skin conditions, vertigo, and weight gain.
Cardiovascular ...Orally, lithium has been reported to cause bradyarrhythmia. A case of symptomatic bradycardia due to sinoatrial node dysfunction is reported in a patient with bipolar disorder who took lithium orotate 20 mg daily for 5 years, despite a serum lithium level in the therapeutic range (111327). Deep vein thrombosis is also reported in 2 patients with bipolar disorder who experienced toxic serum levels of lithium (111329). It is unclear if these effects are a concern with the smaller doses found in lithium supplements.
Dermatologic ...Orally, lithium can cause or exacerbate skin disorders such as hair loss, acne, psoriasis, and rash (9,15,97770). A case of Stevens-Johnson syndrome is also reported in a patient with bipolar disorder who took lithium carbonate at an unknown dose for 17 days (111317).
Endocrine ...Orally, chronic use of lithium has been reported to cause various endocrine disorders. Case reports associate chronic lithium use with hypothyroidism, hyperthyroidism, goiter, hyperparathyroidism, and diabetes insipidus (9,15,104267,104269,104270,104271,111320). In one case report, a 68-year-old male with schizophrenia developed severe hypothyroidism resulting in myxedema coma after taking oral lithium carbonate. He recovered after discontinuation of lithium and administration of levothyroxine. At least two other cases of lithium-associated myxedema coma have been reported (97740). At least 4 cases of lithium-associated hyperparathyroidism have been reported in females aged 53-68 years that had taken lithium for 24 years or more. These patients presented with hypernatremia, hypercalcemia, elevated serum creatinine, thyroid or parathyroid abnormalities, and nephrogenic diabetes insipidus (104269,105344). It is unclear if these effects are a concern with the smaller doses found in lithium supplements.
Gastrointestinal ...Orally, lithium can cause gastrointestinal symptoms. These adverse effects often improve with continued use (9). It is unclear if this effect would occur with the smaller doses found in lithium supplements.
Musculoskeletal ...Orally, lithium can cause muscle weakness. This adverse effect often improves with continued use (9). It is unclear if this effect would occur with the smaller doses found in lithium supplements.
Neurologic/CNS ...Orally, lithium can cause vertigo, muscle weakness, lethargy, fatigue, and a dazed feeling. These adverse effects often improve with continued use. Fine tremor can occur and may persist with continued use. Chronic use of lithium can cause mild cognitive and memory impairment, particularly in the presence of dehydration or hyponatremia (9,15,97745). These long-term neurological adverse effects of lithium are potentially due to accumulation in the central nervous system even when blood levels appear within the therapeutic range (97745). Lithium-associated hyperparathyroidism-induced hypercalcemia has resulted in hallucinations, confusion, insomnia, and agitation (105344). A case of delirium and transient difficulty with word finding is reported in a patient with bipolar disorder treated with lithium 250-500 mg twice daily and 9 sessions of electroconvulsive therapy (111316). A case of mild encephalitis/encephalopathy with a reversible splenial lesion (MERS) is also reported in a patient with bipolar disorder who had a toxic lithium level of 1.94 mEq/L (111318). It is unclear if these effects would occur with the smaller doses found in lithium supplements.
Psychiatric ...Abrupt discontinuation of lithium resulting in a rapid reduction in serum lithium levels can precipitate recurrence of bipolar symptoms (9165). Lithium should be tapered gradually over at least 14 days (9165).
Renal ...Orally, lithium can cause polyuria, polydipsia, and edema (9). Chronic lithium use has been reported to cause central or nephrogenic diabetes insipidus, hypocalciuric hypercalcemia, and nephrotic syndrome (104269,104271,111314,111319). Long-term lithium use is estimated to increase the odds of chronic kidney disease (CKD) by at least 2-fold and may contribute to CKD progression (111315,111324). It is unclear if these effects would occur with the smaller doses found in lithium supplements.
Other ...Orally, chronic use of lithium has been reported to cause an irreversible reduction in taste and smell in a patient with bipolar disorder who took 400-1000 mg/day for 4 months (111313). Chronic use of lithium 1200 mg/day has also been reported to cause dysphagia in a 17-year-old patient with bipolar disorder; however, the patient's serum lithium level was in the toxic range and the adverse effect resolved once the level normalized (111328). It is unclear if these effects would occur with the smaller doses found in lithium supplements.
