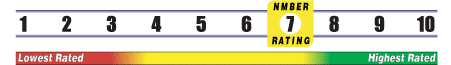
| Ingredients | Not Present |
|---|---|
|
(L. casei )
|
Below is general information about the effectiveness of the known ingredients contained in the product L. Casei. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product L. Casei. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Lacticaseibacillus casei has been safely used alone or in combination with other ingredients in studies lasting up to 8 weeks (90230,112517).
CHILDREN: LIKELY SAFE
when used orally and appropriately in children of most ages.
Lacticaseibacillus casei has been safely used alone in studies lasting up to 4 months (14373,107544). Also, a specific mixture (Latopic, Biomed S.A.) providing 1 billion CFUs of L. casei ŁOCK 0919 50%, Lacticaseibacillus rhamnosus ŁOCK 0900 25%, and L. rhamnosus ŁOCK 0908 25% has been used with apparent safety for 3 months in children under 2 years of age (107510). In addition, in children ages 4-17 years, a 1:1:1 combination of L. casei CECT 9104, Bifidobacterium animalis subsp. lactis CECT 8145, and Bifidobacterium longum CECT 7347 providing 1 billion CFUs has been used with apparent safety for 12 weeks (107531). There is insufficient reliable information available about the safety of L. casei in preterm infants with a birth weight under 1000 grams. Cases of bacteremia have occurred rarely in preterm infants given other probiotics (102416,111610,111612,111613,111850,111852,111853). The US Food and Drug Administration (FDA) has issued a warning about cases of serious infections caused by probiotics reported in very preterm or very low birth weight infants under 1000 grams (111610). Similarly, the American Academy of Pediatrics does not support the routine administration of probiotics to these infants due to conflicting data on safety and efficacy (111608).
PREGNANCY: POSSIBLY SAFE
when used orally and appropriately.
A combination of Lacticaseibacillus casei, Lactobacillus acidophilus, and Bifidobacterium bifidum has been used with apparent safety for 6 weeks, starting at 24-28 weeks' gestation (95416,98430).
LACTATION:
There is insufficient reliable information available about the safety of Lacticaseibacillus casei during lactation.
However, there are currently no reasons to expect safety concerns when used appropriately.
Below is general information about the interactions of the known ingredients contained in the product L. Casei. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, taking Lacticaseibacillus casei with antibiotic drugs might decrease the effectiveness of L. casei.
L. casei preparations usually contain live and active organisms. Therefore, simultaneously taking antibiotics might kill a significant number of the organisms (1740). Tell patients to separate administration of antibiotics and L. casei preparations by at least two hours.
|
Below is general information about the adverse effects of the known ingredients contained in the product L. Casei. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, Lacticaseibacillus casei is generally well tolerated.
Most Common Adverse Effects:
Orally: Mild gastrointestinal adverse effects.
Serious Adverse Effects (Rare):
Orally: There is concern that lactobacilli may cause infections in some people.
Gastrointestinal ...Orally, taking Lacticaseibacillus casei in combination with other probiotics may cause gastrointestinal side effects including abdominal pain (90291); however, these events are uncommon.
Immunologic
...Since Lacticaseibacillus casei preparations contain live and active microorganisms, there is some concern that they might cause pathogenic infection in some patients.
Some lactobacilli species have been isolated in some cases of bacteremia, sepsis, splenic abscess, endocarditis, aortic dissection, necrotizing fasciitis, pancreatic necrosis, and meningoencephalitis. Most of these cases are thought to be due to the translocation of bacteria from other locations in the body in which they occur naturally, such as the oral cavity and gastrointestinal tract. The majority of cases are not related to the use of probiotic supplements and most are not associated with the use of L. casei (107543,112516). There is at least one case of L. casei bacteremia and endocarditis thought to be related with L. casei intake in a 71-year-old immunocompromised female (112520).
There are two cases of L. casei infection in a prosthetic joint (90282,112514). In one case, the 95-year-old female with a history of hypertension, diabetes, and heart disease was known to consume yogurt containing L. casei. However, it was not confirmed that the infection was related to the consumption of this product. Spread from the gastrointestinal tract or vaginal flora could not be ruled out (90282). In the case of an 80-year-old male, the cause was unknown as there was no probiotic supplementation and no underlying medical condition or infectious portal of entry (112514).
A specific probiotic preparation (NBL probiotic ATP, Nobel) containing L. casei, Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum, Bifidobacterium animalis subsp. lactis, fructo-oligosaccharides, galacto-oligosaccharides, colostrum, and lactoferrin was found to be a significant risk factor for vancomycin-resistant Enterococcus colonization in premature infants. Although there was no direct link to determine causation, it was hypothesized that the probiotic mixture helped to mediate the acquisition and transfer of antibiotic resistance genes (96890).
