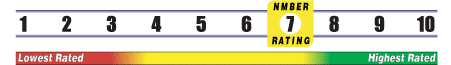
Below is general information about the effectiveness of the known ingredients contained in the product Neem Powder. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Neem Powder. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
POSSIBLY SAFE ...when neem bark extract is used orally and appropriately, short-term. Neem bark extract has been used safely in clinical research at doses up to 60 mg daily for up to 10 weeks (12822). ...when neem leaf and twig extract is used orally and appropriately, short-term. Neem leaf and twig extract has been used safely in clinical research at doses up to 500 mg twice daily for up to 12 weeks (104181). ...when neem leaf extract gel is used intraorally for up to 6 weeks (12824,64845,64850,94567). ...when neem oil, cream, or face wash is used topically on the skin for up to 2 weeks (64876,64878,64882,102867,107883).
POSSIBLY UNSAFE ...when neem or neem oil is used orally in large amounts or long-term. Preliminary clinical research suggests neem might be toxic to the kidneys or liver with high-dose or chronic use. Cardiac arrest has also been reported (12835,64870,64873).
CHILDREN: POSSIBLY SAFE
when neem extract is used topically.
It has been used with apparent safety as a shampoo, with one or two total applications (97928).
CHILDREN: LIKELY UNSAFE
when neem oil or seeds are used orally.
There are reports of infants who were severely poisoned and died after oral use of neem (3473,3474,3476,64855,64875).
PREGNANCY: LIKELY UNSAFE
when neem oil or leaf is used orally.
Neem oil and leaf have been used as abortifacients (12825,12835,64884,64889).
LACTATION:
Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product Neem Powder. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Neem might increase the risk of hypoglycemia when taken with antidiabetes drugs.
|
Theoretically, neem leaf extract might increase the levels and clinical effects of CYP2C8 substrates.
In vitro research shows that neem leaf methanol extract inhibits CYP2C8 enzymes (111593). So far, this reaction has not been reported in humans.
|
Theoretically, neem leaf extract might increase the levels and clinical effects of CYP2C9 substrates.
In vitro research shows that neem leaf methanol extract inhibits CYP2C9 enzymes (111593). So far, this reaction has not been reported in humans.
|
Theoretically, neem leaf extract might increase the levels and clinical effects of CYP3A4 substrates.
In vitro research shows that neem leaf methanol extract inhibits CYP3A4 enzymes (111593). So far, this reaction has not been reported in humans.
|
Theoretically, neem might decrease the effectiveness of immunosuppressants.
Animal research suggests that neem might have immunostimulant effects (12825).
|
Theoretically, neem leaf extract might increase the levels and clinical effects of P-glycoprotein substrates.
In vitro research shows that neem leaf methanol extract inhibits renal P-glycoprotein transport activity (107850). So far, this reaction has not been reported in humans.
|
Below is general information about the adverse effects of the known ingredients contained in the product Neem Powder. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, neem extracts seem to be well tolerated in adults.
However, high-quality assessment of safety has not been conducted. In children, oral use of neem oil can cause serious adverse effects. Topically, neem seems to be well tolerated in children and adults.
Most Common Adverse Effects:
Topically: Contact dermatitis in sensitive individuals.
Serious Adverse Effects (Rare):
Orally: Cardiac arrest, nephrotoxicity, and ventricular fibrillation with neem leaf in adults. Encephalopathy, hematologic abnormalities, hepatotoxicity, and nephrotoxicity with neem oil in infants and young children.
Cardiovascular ...Orally, neem leaf has been reported to cause ventricular fibrillation and cardiac arrest after ingestion in humans (64873,64870).
Dental ...Topically, use of neem twigs to brush teeth, which is a traditional dental hygiene practice in India, has been associated with vitiligo of the lips. The limonoid constituents in neem, which have been shown to inhibit melanogenesis and have cytotoxic effects, combined with repeated, local trauma from this dental hygiene practice are thought to cause this leucodermic reaction. In a case series of seven patients experiencing vitiligo of the lips from neem twigs, use of toothpaste and topical tacrolimus along with avoidance of neem stopped the progression of depigmentation in all patients. Repigmentation was reported in four of the seven patients 12 months after discontinuing neem-based dental hygiene practices (100958).
Dermatologic ...Topically, neem products have been associated with dermatologic reactions. Some case reports have associated the use of topical neem oil with contact dermatitis (64851,94568,102867). In one case series, the topical application of neem seed extract shampoo was associated with skin irritation, red spots, and a burning feeling of the scalp (64848). Use of neem twigs to brush teeth, which is a traditional dental hygiene practice in India, has been associated with vitiligo of the lips. The limonoid constituents in neem, which have been shown to inhibit melanogenesis and have cytotoxic effects, combined with repeated, local trauma from this dental hygiene practice are thought to cause this leucodermic reaction. In a case series of seven patients experiencing vitiligo of the lips from neem twigs, use of toothpaste and topical tacrolimus along with avoidance of neem stopped the progression of depigmentation in all patients. Repigmentation was reported in four of the seven patients 12 months after discontinuing neem-based dental hygiene practices (100958).
Gastrointestinal ...Orally, neem oil has been reported to cause vomiting and loose stools in infants and small children (3473,3474,3476,64865).
Genitourinary ...Orally, neem leaf has been reported to cause oliguria and anuria in adults (12833,12834). After a single intrauterine instillation, purified neem oil has been reported to cause endometritis in healthy, tubectomised females (64886).
Hematologic
...Orally, neem leaf has been reported to cause hemolysis in adults (12835).
In one case report, a 35-year-old male with diabetes and glucose-6-phosphate dehydrogenase (G6PD) deficiency developed hemolytic anemia and jaundice after drinking several liters of neem tea daily for 3 weeks. All symptoms resolved after discontinuation and supportive treatment (94571). Orally, neem oil has been reported to cause metabolic acidosis, anemia, and polymorphonuclear leukocytosis in infants and young children (3473,3474,3476,64865).
A single intrauterine instillation of purified neem oil has been reported to cause mild transient eosinophilia in healthy, tubectomised females (64886).
Hepatic ...Orally, neem oil has been associated with reports of hepatotoxicity in infants and children. These adverse effects occurred after single doses of neem oil ranging from a few drops to 60 mL. Pathologic findings on liver biopsy reports have been consistent with Reye-like syndrome (3473,3474,3475).
Immunologic ...Topically, a case of aggravated bullous pemphigoid requiring hospitalization is reported in a 47-year-old patient with this autoimmune condition after application of neem oil to blisters for an unknown duration (111715).
Neurologic/CNS ...Orally, single doses of neem oil ranging from a few drops to 60 mL have been associated with reports of encephalopathy in infants and small children. Symptoms include drowsiness, seizure, loss of consciousness, coma, cerebral edema, Reye-like syndrome, and death within hours of ingestion (3473,3474,3476,3476,64855,94750). There is also at least one case report of neurotoxicity in an adult after ingestion of a neem-based pesticide. A 35-year-old female experienced neurotoxicity requiring intensive medical care and ventilation after ingestion of a pesticide containing azadirachtin, a constituent of neem oil (64858).
Ocular/Otic ...In one case report, a 35-year-old female developed toxic optic neuropathy and vision loss in both eyes lasting for two days after consuming 150 mL of neem oil in a suicide attempt five days earlier (64856).
Renal ...Orally, neem leaf has been reported to cause oliguria, anuria, acute tubular necrosis, and nephrotoxicity in adults (12833,12834). There are some case reports of children developing Reye-like syndrome after ingestion of neem oil. Pathologic findings on renal biopsy reports have been consistent with Reye syndrome (3473,3474,3475).
