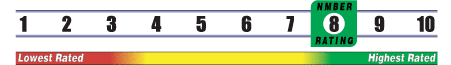
| Ingredients | Not Present |
|---|---|
|
(B. lactis )
|
Below is general information about the effectiveness of the known ingredients contained in the product B. Lactis. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product B. Lactis. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Bifidobacterium lactis has been safely used alone or in combination with other probiotics in clinical trials lasting up to 12 weeks (92255,98502,105158,107572,107581,107586,110979,110985,110986,110992)(110993,110998,110999).
CHILDREN: LIKELY SAFE
when used orally and appropriately in children of most ages.
Bifidobacterium lactis has been safely used alone or in combination with other probiotics in infants and children for up to 15 months (3169,3458,92265,95381,95382,98736,105149,107582,107583,107585)(107587,107590,110984,110987,110988,110991,110994,110995). A combination probiotic containing B. lactis and Lactobacillus acidophilus (HOWARU Protect, Danisco) has been used safely for up to 6 months in children aged 3-5 years (16847). A specific combination of B. lactis, Bifidobacterium bifidum, and L. acidophilus (Complete Probiotic Platinum) has also been used safely for up to 18 months in children aged 4 months to 5 years (103436). In addition, in children ages 4-17 years, 1 billion CFUs of a 1:1:1 combination of B. lactis CECT 8145, Lacticasebacillus casei CECT 9104, and Bifidobacterium longum CECT 7347 has been used safely for 12 weeks (107531). There is insufficient reliable information available about the safety of B. lactis in preterm infants with a birth weight under 1000 grams. Cases of bacteremia have occurred rarely in preterm infants given other probiotics (102416,111610,111612,111613,111850,111852,111853). The US Food and Drug Administration (FDA) has issued a warning about cases of serious infections caused by probiotics reported in very preterm or very low birth weight infants under 1000 grams (111610). Similarly, the American Academy of Pediatrics does not support the routine administration of probiotics to these infants due to conflicting data on safety and efficacy (111608).
PREGNANCY AND LACTATION:
Insufficient reliable information available.
A meta-analysis of four clinical trials shows that taking probiotics during pregnancy increases the relative risk of pre-eclampsia by 85% when compared with placebo. Although the specific effects of Bifidobacterium lactis are unclear from this analysis, three of the included studies used B. lactis in combination with Lacticaseibacillus rhamnosus (105185). More information is needed to determine if certain patients are at increased risk.
Below is general information about the interactions of the known ingredients contained in the product B. Lactis. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, taking Bifidobacterium lactis with antibiotic drugs might decrease the effectiveness of B. lactis.
|
Below is general information about the adverse effects of the known ingredients contained in the product B. Lactis. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, Bifidobacterium lactis seems to be well tolerated by most patients.
Most Common Adverse Effects:
Orally: Diarrhea.
Serious Adverse Effects (Rare):
Orally: There is concern that probiotics may cause infections in some people.
Dermatologic ...In clinical research, two cases of rash, one with itching, were reported by patients taking a combination of Bifidobacterium lactis BB-12, Lacticaseibacillus paracasei F19, and Lactobacillus acidophilus La5. However, it is not clear if these adverse effects were due to B. lactis, other probiotics, or the combination, or if the events were idiosyncratic (90236).
Gastrointestinal ...Bloating and flatulence have been reported with probiotic use; however, these adverse effects have not been reported from ingestion of Bifidobacterium lactis in particular. When taken orally, B. lactis can cause diarrhea and other gastrointestinal complaints in children (3169,95381,105149). Gastrointestinal complaints including worsening diarrhea, abdominal pain, constipation, stomach burn, and flatulence have been reported rarely (110986,110999).
Immunologic
...There have been cases of Bifidobacterium bacteremia in critically ill patients (102416,107599).
These cases are rare and none seem to be due to Bifidobacterium lactis alone.
A specific preparation (NBL probiotic ATP, Nobel) containing B. lactis, Lacticaseibacillus casei, Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum, fructo-oligosaccharides, galacto-oligosaccharides, colostrum, and lactoferrin was found to be a significant risk factor for vancomycin-resistant Enterococcus colonization in premature infants. Although there was no direct link to determine causation, it was hypothesized that the probiotic mixture helped to mediate the acquisition and transfer of antibiotic resistance genes (96890).
