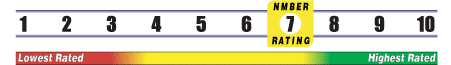
| Ingredients | Amount per serving |
|---|---|
|
(from Sweet Wormwood)
(Artemisinin (Form: from sweet wormwood Genus: Artemisia Species: annua) )
|
100 mg |
Modified Cellulose Note: vegetarian capsule, Cellulose, Magnesium Stearate Note: vegetable source
Below is general information about the effectiveness of the known ingredients contained in the product Best Artemisinin. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Best Artemisinin. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
POSSIBLY SAFE ...when used orally and appropriately, short-term. Sweet Annie 300 mg daily has been used with apparent safety in studies lasting up to 9 months (11055,94520,94521). Sweet Annie tea, prepared from dried leaves and twigs and consumed in divided doses daily, has been used with apparent safety for up to 7 days (11055,11058). While rare, there is some concern that Sweet Annie might cause liver damage (16895,103254,103255).
POSSIBLY SAFE ...when used sublingually and appropriately, short-term. Sweet Annie up to 2400 biological units daily as sublingual immunotherapy has been used with apparent safety in studies lasting up to 16 months (106441,112392,112393,112394). There is insufficient reliable information available about the safety of Sweet Annie when used topically.
PREGNANCY AND LACTATION:
Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product Best Artemisinin. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Sweet Annie may alter plasma levels and clinical effects of drugs metabolized by CYP2B6.
In vitro research shows that the Sweet Annie constituent artemisinin induces CYP2B6, possibly increasing CYP2B6 activity by 1.6-fold (92501,109316). However, Sweet Annie extract seems to inhibit the activity of CYP2B6 in vitro, suggesting that other constituents of Sweet Annie play a role in its effects on the overall activity of this enzyme (109316). More information is needed to determine whether taking Sweet Annie extract affects the metabolism of CYP2B6 substrates.
|
Sweet Annie may alter plasma levels and clinical effects of drugs metabolized by CYP3A4.
In vitro research shows that the Sweet Annie constituent artemisinin induces CYP3A4, possibly increasing CYP3A4 activity by 1.9-fold (92501). However, Sweet Annie extract seems to inhibit the activity of CYP3A4 in vitro, suggesting that other constituents of Sweet Annie play a role in its effects on the overall activity of this enzyme (109316). More information is needed to determine whether taking Sweet Annie extract affects the metabolism of CYP3A4 substrates.
|
Theoretically, concomitant use might have additive adverse hepatotoxic effects.
|
Below is general information about the adverse effects of the known ingredients contained in the product Best Artemisinin. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, Sweet Annie is generally well-tolerated.
Most Common Adverse Effects:
Orally: Nausea and vomiting.
Serious Adverse Effects (Rare):
Orally: Hepatotoxicity.
Gastrointestinal ...Orally, Sweet Annie might cause gastrointestinal upset including nausea and vomiting in some patients (11058,112393).
Hepatic
...Orally, Sweet Annie might cause hepatic adverse effects (16895,103254,103255).
In one case, a 52-year-old patient developed hepatitis after taking the Sweet Annie constituent artemisinin 200 mg three times daily for 10 days. The patient developed abdominal pain and dark urine and was found to have elevated liver enzymes consistent with hepatitis. Symptoms resolved within 2 weeks of discontinuing use. Although it is possible this supplement caused liver disease in this patient, it is not certain. In clinical trials evaluating artemisinin, elevated liver enzymes have only been reported in around 0.9% of patients. However, the dose of artemisinin in this case was substantially higher than a typical dose (16895). A case of severe acute cholestatic hepatitis has also been reported in a 51-year-old male who drank Sweet Annie tea daily, prepared using 1.25 grams of Sweet Annie powder, for malaria prophylaxis during a 4-week trip to Ethiopia. Three weeks after his return, he presented with malaise, abdominal discomfort, jaundice, elevated liver enzymes, and markers of cholestasis. The patient was treated with corticosteroids and ursodeoxycholic acid and ultimately recovered (103255).
A series of cases linking the use of a supercritical carbon dioxide extract of Sweet Annie to hepatoxicity has also been reported. Of the 29 reports of adverse hepatic reactions to this extract, 19 patients noted symptoms within 12 weeks of starting the extract, 16 patients experienced jaundice, and 9 patients required hospitalization. Other common symptoms of hepatotoxicity included abdominal pain, vomiting, nausea, fever, headache, anorexia, malaise, fatigue, and lethargy. All but one case involved doses below or up to the extract's recommended dose of 300 mg daily. Upon discontinuation, symptoms resolved completely or were improved in nearly all cases (103254).
Immunologic ...One case of a mild allergic reaction to Sweet Annie tea has been reported. The reaction was characterized by a rash and cough that resolved quickly and did not require treatment (11059). When low doses are taken sublingually by individuals allergic to Sweet Annie, numbness of the tongue and throat itching have been reported (109315,112392,112393,112394).
