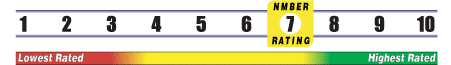
| Ingredients | Amount Per Serving |
|---|---|
|
(Monascus purpureus )
(1.2 g)
(Red Yeast Rice powder Genus: Monascus Species: purpureus Note: 1.2 g )
|
1200 mg |
Gelatin (Form: Bovine), Contains <2% of (Form: Rice Flour, Silica, Vegetable Magnesium Stearate)
Below is general information about the effectiveness of the known ingredients contained in the product Red Yeast Rice 600 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Red Yeast Rice 600 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
POSSIBLY SAFE ...when used orally and appropriately. Red yeast rice 1.2 grams daily has been used with apparent safety in clinical studies for up to 4.5 years (512,2624,6988,6995,6996,17089,18110,70508,70513) (70520,70525,70530,95664,95666). However, red yeast rice products can contain an HMG-CoA reductase inhibitor identical to lovastatin, and can cause the same side effects as this drug. It is recommended that people taking red yeast rice products be monitored for the same hepatic and muscle-related adverse effects that are seen with lovastatin (98822).
PREGNANCY: LIKELY UNSAFE
when used orally.
The red yeast rice constituent, lovastatin, has induced fetal skeletal malformations in animals (2619). The US Food and Drug Administration (FDA) recommends that most patients discontinue statin therapy during pregnancy due to the risks to the fetus; however, in certain high-risk patients, a prescription statin may be continued during pregnancy (107954).
LACTATION:
Insufficient reliable information available; avoid using.
The US FDA recommends against breastfeeding while taking statins (107954).
Below is general information about the interactions of the known ingredients contained in the product Red Yeast Rice 600 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, taking red yeast rice in combination with cyclosporine might increase the risk of myopathy.
|
Theoretically, drugs that inhibit the CYP3A4 enzymes might increase levels of lovastatin from red yeast rice.
Red yeast rice contains varying levels of the statin drug lovastatin, which is metabolized by CYP3A4 (104951). Combining red yeast rice with CYP3A4 inhibitors might increase serum levels of lovastatin from red yeast rice.
|
Theoretically, taking red yeast rice in combination with gemfibrozil might increase the risk of rhabdomyolysis.
|
Theoretically, concomitant use might increase the risk of liver damage.
Red yeast rice contains varying levels of the drug lovastatin. Lovastatin can cause liver damage in some people (104951). Some clinical research suggests that supplements containing red yeast rice might increase liver enzyme levels in some, but not all, participants (42692,70491). Cases of acute hepatitis have been associated with red yeast rice (16654,54477). Combining it with hepatotoxic drugs might further increase this risk.
|
Theoretically, taking red yeast rice with other statins might increase the risk of potential adverse effects.
Red yeast rice contains varying levels of the statin drug lovastatin and might result in supratherapeutic levels when used with other statins. Based on evaluation of data from the US Food and Drug Administration's adverse event reporting system (FAERS), it is recommended that red yeast rice products be avoided in people taking prescription statins (98822).
|
Theoretically, taking red yeast rice in combination with high-dose niacin might increase the risk of rhabdomyolysis.
|
Below is general information about the adverse effects of the known ingredients contained in the product Red Yeast Rice 600 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, red yeast rice seems to be well tolerated.
Most Common Adverse Effects:
Orally: Abdominal discomfort, diarrhea, dizziness, flatulence, headache, heartburn, myopathy, and nausea.
Serious Adverse Effects (Rare):
Orally: There have been reports of hepatotoxicity and rhabdomyolysis, likely related to the lovastatin content of red yeast rice. Contaminated red yeast rice might cause renal toxicity.
Cardiovascular ...Orally, red yeast rice used in combination with other natural ingredients, such as green tea extract and policosanol, has been associated with a case of chest pain and a case of tachycardia requiring hospitalization, in post marketing surveillance (94001).
Dermatologic ...Orally, red yeast rice has been rarely associated with mild cases of pruritus and rash in clinical trials and post marketing surveillance (70531,94001,95664). Two cases of alopecia were reported in patients taking red yeast rice in clinical research (17089).
Gastrointestinal ...Orally, red yeast rice has been associated with mild adverse effects including abdominal discomfort, bloating, heartburn, flatulence, diarrhea or loose stools, nausea, vomiting, abdominal distention or pain, and reduced appetite, in clinical trials and post marketing surveillance (2624,6988,16836,70556,94001,95664). Taking red yeast rice with food may reduce the risk of heartburn, gas, and abdominal discomfort.
Genitourinary
...Orally, red yeast rice has been associated with rare reports of erectile dysfunction (70520).
In one case report, a 39-year-old male developed erectile dysfunction after taking red yeast rice for one week. The dysfunction resolved after discontinuation of red yeast rice (98822).
A case of cystitis has been reported in a patient taking a specific combination product (Limicol, Laboratoire Lescuyer) containing red yeast rice extract, sugar cane extract, dry artichoke leaf extract, dry garlic extract, pine bark extract, vitamin E, riboflavin, and inositol hexanicotinate (89451). However, it is unclear if this event was associated with red yeast rice or other ingredients in the supplement.
Hepatic ...Orally, red yeast rice preparations have been linked to case reports of hepatotoxicity, including increased liver enzymes and acute hepatitis (16654,54477,94001,95664,98822,112644). Since red yeast rice often contains significant concentrations of the statin-like monacolin constituents, including lovastatin, it has the potential to cause similar side effects, including elevated liver enzymes. Clinical trials have shown that red yeast rice intake is associated with mild increases in levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which suggests possible liver damage (42692,70491,70513,70531,70547,107952). A case report describes a 62-year-old female who developed mixed hepatocellular and cholestatic hepatitis while taking red yeast rice. Signs and symptoms included fever, dark colored urine, weight loss, hyperbilirubinemia, and elevated ALT levels, all of which resolved after stopping red yeast rice (112089). A small study in various patient populations shows that taking a specific combination product (Armolipid Plus, Rottapharm S.p.A.) containing red yeast rice, berberine, policosanol, and other ingredients modestly increases levels of ALT, but not AST (107953). Clinical reviews of red yeast rice products show the risk of liver injury is comparable to the placebo or active control group, including pravastatin or lovastatin, when taken for up to 24 weeks (95664,95666).
Immunologic
...In one case report, a 58-year-old male presented with complaints of chronic dysphagia from eosinophilic esophagitis 12 months after starting an oral red yeast rice supplement (Artechol) containing monacolin K.
Eosinophilic esophagitis resolved after cessation of red yeast rice (104465).
Inhalation of red yeast rice powder has resulted in one case of anaphylaxis (6997).
Musculoskeletal ...Orally, red yeast rice preparations have been linked to cases of myalgia, muscle spasm, rhabdomyolysis, and myopathy (9587,15017,16654,16834,16836,17089,70475,94001,95664,98822)(103311,112644,112645). Also, elevated creatine kinase levels up to 10 times normal, suggesting muscle injury and inflammation, have been reported in clinical and post-marketing research reports (6988,9587,15017,42692,70530,70567,94001,95664). The risk of muscle injury with red yeast rice seems to be similar to that with statins. In a small 3-month clinical trial in patients with previous statin intolerance, the rate of therapy discontinuation due to myalgia was similar between patients taking a specific red yeast rice product (Red Yeast Rice, Sylvan Bioproducts) 2400 mg twice daily (containing a daily dose of about 10 mg lovastatin) and patients taking pravastatin 20 mg twice daily (17089). However, in one case report, a 53-year-old patient experienced myalgia after 4 months of taking a red yeast rice product containing 4-8 mg lovastatin. Another case report describes a 50-year-old female who developed generalized myalgias and rhabdomyolysis, with elevated creatine phosphokinase, lactate dehydrogenase, and myoglobin levels, while taking red yeast rice (112306). The risk of myopathy seems to depend on the specific red yeast rice formulation and dose used (95903).
Neurologic/CNS ...Orally, red yeast rice has been associated with dizziness, headache, fatigue, and tingling in the extremities (6988,16836,17089,18110,94001). A case of peripheral neuropathy occurred in a 60-year-old male with a gastrointestinal tumor who was taking imatinib 400 mg daily along with red yeast rice for 3 years (89453). Three months after cessation of red yeast rice, symptoms resolved.
Ocular/Otic ...Orally, red yeast rice in combination with policosanol has been associated with one post-marketing report of hazy vision (94001).
Renal ...Orally, red yeast rice contaminated with citrinin may cause renal toxicity. Analyses of red yeast rice products have found that about one-third to two-thirds of these products contain citrinin (9588,17501,95666). Citrinin is a nephrotoxin that results from incorrect rice fermentation processes (9588,17501,70543). In vitro and in animal research, citrinin has been reported to cause kidney damage (70482,70542,70540).
Other ...Orally, red yeast rice has been associated with rare cases of edema (70508,70520,70525).
