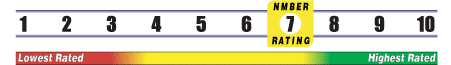
| Ingredients | Amount Per Serving |
|---|---|
|
(contains)
(Lutigold Lutein Note: contains )
|
6 mg |
| 240 mcg |
Safflower Oil, Gelatin, Vegetable Glycerin, yellow Beeswax
Below is general information about the effectiveness of the known ingredients contained in the product Lutigold Lutein 6 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Lutigold Lutein 6 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Consuming up to 20 mg of lutein daily from both dietary and supplemental sources appears to be safe (3219,3220,60167). Lutein supplements have been safely used in clinical trials at doses of up to 20 mg daily for up to 10 years (11798,60133,60177,94703,94701,100986,104570,107107,108615,109763).
CHILDREN: LIKELY SAFE
when used orally and appropriately.
A specific product containing lutein (LUTEINofta, SOOFT Italia SpA) has been used with apparent safety in infants at a dose of 0.14 mg daily for 36 weeks (91163).
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally and appropriately in amounts found in foods.
The high end of dietary lutein intake ranges from 6.9-11.7 mg/day (3219,3220).
LIKELY SAFE ...when used orally and appropriately in doses of up to 2 mg daily. Zeaxanthin supplements have been safely used in clinical trials at doses of up to 2 mg daily for up to 10 years (94701,94702,94703,108615).
POSSIBLY SAFE ...when used orally and appropriately in amounts greater than 2 mg daily. Zeaxanthin supplements in doses of 8-10 mg daily for up to 12 months have been used with apparent safety in clinical trials (60175,60245).
CHILDREN: POSSIBLY SAFE
when used orally and appropriately.
A specific product containing zeaxanthin (LUTEINofta, SOOFT Italia SpA) has been used with apparent safety in infants at a dose of 0.0006 mg daily for 36 weeks (91163). There is insufficient reliable information available about the safety of zeaxanthin at higher doses or in older children.
PREGNANCY AND LACTATION: LIKELY SAFE
when used orally and appropriately in amounts found in foods.
Zeaxanthin is found in breast milk and levels correlate with infant status (106365). There is insufficient reliable information available about the safety of supplemental zeaxanthin.
Below is general information about the interactions of the known ingredients contained in the product Lutigold Lutein 6 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, taking zeaxanthin with antidiabetes drugs might increase the risk of hypoglycemia.
|
Below is general information about the adverse effects of the known ingredients contained in the product Lutigold Lutein 6 mg. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General ...Orally, dietary and supplemental lutein is generally well tolerated. Doses up to 20 mg daily have not resulted in adverse effects.
General ...Orally, dietary and supplemental zeaxanthin are generally well tolerated. No adverse effects have been reported in clinical research.
