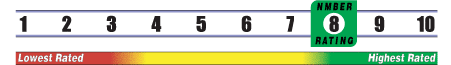
Non-GMO Sodium Hyaluronate .
Brand name products often contain multiple ingredients. To read detailed information about each ingredient, click on the link for the individual ingredient shown above.
This is a "branded ingredient." This means that it is an ingredient used in other companies' products. For example, this product might be just one ingredient used in another company's product that contains multiple ingredients. These branded ingredients usually are not available for sale as stand alone products for consumers.
Below is general information about the effectiveness of the known ingredients contained in the product HyaMax. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product HyaMax. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE ...when used orally and appropriately. Supplements standardized to contain hyaluronic acid 70%, in an 80 mg daily dose, have been used daily for up to 3 months with no reports of adverse effects (55742,91779). ...when used topically and appropriately. Hyaluronic acid, in a gel or impregnated gauze, has been safely applied to the skin in clinical trials (7889,7892,104389,108627,108640). ...when eye drop preparations containing up to 0.3% hyaluronic acid are used multiple times per day for up to 3 months (97885,97894,97895,110555).
PREGNANCY:
There is insufficient reliable information available about the safety of hyaluronic acid; avoid using.
LACTATION:
There is insufficient reliable information available about the safety of hyaluronic acid.
It is not known if hyaluronic acid is excreted in breast milk (7890); avoid using.
Below is general information about the interactions of the known ingredients contained in the product HyaMax. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Below is general information about the adverse effects of the known ingredients contained in the product HyaMax. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally and topically, hyaluronic acid appears to be well tolerated.
Most Common Adverse Effects:
Topically: Eczema, erythema, itching, wound hemorrhage, wound infection (e.g., erysipelas).
Dermatologic
...The use of needle-free devices to inject hyaluronic acid for cosmetic purposes has been reported to cause serious injury, and in some cases permanent harm, to the skin, lips, and eyes (108613).
Topically, hyaluronic acid application has been reported to cause eczema, erythema, itching, wound hemorrhage, and wound infection (e.g., erysipelas) (108628,108640).
Ocular/Otic ...Ocular pain has been reported rarely in patients using eye drops containing up to 0. 3% hyaluronic acid (97885).
