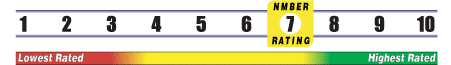
| Ingredients | Amount Per Serving |
|---|---|
|
(root)
(1:5 in 50% Alcohol)
(Ashwagandha (Form: 1:5 in 50% Alcohol) PlantPart: root )
|
1.23 mL |
organic Cane Sugar Alcohol, purified Water Note: pH neutral, organic Ashwagandha PlantPart: root Genus: Withania Species: somnifera
Below is general information about the effectiveness of the known ingredients contained in the product Ashwagandha. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Ashwagandha. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
POSSIBLY SAFE ...when used orally and appropriately, short-term. Ashwagandha has been used with apparent safety in doses of up to 1250 mg daily for up to 6 months (3710,11301,19271,90649,90652,90653,97292,101816,102682,102683) (102684,102685,102687,103476,105824,109586,109588,109589,109590). ...when used topically. Ashwagandha lotion has been used with apparent safety in concentrations up to 8% for up to 2 months (111538).
PREGNANCY: LIKELY UNSAFE
when used orally.
Ashwagandha has abortifacient effects (12).
LACTATION:
Insufficient reliable information available; avoid using.
Below is general information about the interactions of the known ingredients contained in the product Ashwagandha. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, taking ashwagandha with antidiabetes drugs might increase the risk of hypoglycemia.
|
Theoretically, taking ashwagandha with antihypertensive drugs might increase the risk of hypotension.
Animal research suggests that ashwagandha might lower systolic and diastolic blood pressure (19279). Theoretically, ashwagandha might have additive effects when used with antihypertensive drugs and increase the risk of hypotension.
|
Theoretically, taking ashwagandha might increase the sedative effects of benzodiazepines.
There is preliminary evidence that ashwagandha might have an additive effect with diazepam (Valium) and clonazepam (Klonopin) (3710). This may also occur with other benzodiazepines.
|
Theoretically, taking ashwagandha might increase the sedative effects of CNS depressants.
Ashwagandha seems to have sedative effects. Theoretically, this may potentiate the effects of barbiturates, other sedatives, and anxiolytics (3710).
|
Theoretically, ashwagandha might decrease the levels and clinical effects of CYP1A2 substrates.
In vitro research shows that ashwagandha extract induces CYP1A2 enzymes (111404).
|
Theoretically, ashwagandha might decrease the levels and clinical effects of CYP3A4 substrates.
In vitro research shows that ashwagandha extract induces CYP3A4 enzymes (111404).
|
Theoretically, taking ashwagandha with hepatotoxic drugs might increase the risk of liver damage.
|
Theoretically, taking ashwagandha might decrease the effects of immunosuppressants.
|
Ashwagandha might increase the effects and adverse effects of thyroid hormone.
Concomitant use of ashwagandha with thyroid hormones may cause additive therapeutic and adverse effects. Preliminary clinical research and animal studies suggest that ashwagandha boosts thyroid hormone synthesis and secretion (19281,19282,97292). In one clinical study, ashwagandha increased triiodothyronine (T3) and thyroxine (T4) levels by 41.5% and 19.6%, respectively, and reduced serum TSH levels by 17.4% from baseline in adults with subclinical hypothyroidism (97292).
|
Below is general information about the adverse effects of the known ingredients contained in the product Ashwagandha. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, ashwagandha seems to be well-tolerated.
Topically, no adverse effects have been reported. However, a thorough evaluation of safety outcomes has not been conducted.
Most Common Adverse Effects:
Orally: Diarrhea, gastrointestinal upset, nausea, and vomiting. However, these adverse effects do not commonly occur with typical doses.
Serious Adverse Effects (Rare):
Orally: Some case reports raise concerns about acute hepatitis, acute liver failure, hepatic encephalopathy, the need for liver transplantation, and death due to liver failure with ashwagandha treatment.
Dermatologic ...Orally, dermatitis has been reported in three of 42 patients in a clinical trial (19276).
Endocrine ...A case report describes a 73-year-old female who had taken an ashwagandha root extract (unspecified dose) for 2 years to treat hypothyroidism which had been previously managed with levothyroxine. The patient was diagnosed with hyperthyroidism after presenting with supraventricular tachycardia, chest pain, tremor, dizziness, fatigue, irritability, hair thinning, and low thyroid stimulating hormone (TSH) levels. Hyperthyroidism resolved after discontinuing ashwagandha (108745). Additionally, an otherwise healthy adult who was taking ashwagandha extract orally for 2 months experienced clinical and laboratory-confirmed thyrotoxicosis. Thyrotoxicosis resolved 50 days after discontinuing ashwagandha, without other treatment (114111).
Gastrointestinal ...Orally, large doses may cause gastrointestinal upset, diarrhea, and vomiting secondary to irritation of the mucous and serous membranes (3710). When taken orally, nausea and abdominal pain (19276,110490,113609) and gastritis and flatulence (90651) have been reported.
Genitourinary ...In one case report, a 28-year-old male with a decrease in libido who was taking ashwagandha 5 grams daily over 10 days subsequently experienced burning, itching, and skin and mucous membrane discoloration of the penis, as well as an oval, dusky, eroded plaque (3 cm) with erythema on the glans penis and prepuce (32537).
Hepatic ...Orally, ashwagandha in doses of 154 mg to 20 grams daily has played a role in several case reports of cholestatic, hepatocellular, and mixed liver injuries. In most of these cases, other causes of liver injury were excluded, and liver failure did not occur. Symptoms included jaundice, pruritus, malaise, fatigue, lethargy, weight loss, nausea, diarrhea, abdominal pain and distension, stool discoloration, and dark urine. Symptom onset was typically 5-180 days from first intake, although in some cases onset occurred after more than 12 months of use (102686,107372,110490,110491,111533,111535,112111,113610,114113). Laboratory findings include elevated aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase, serum bilirubin, and international normalized ratio (INR) (112111,113610,114113). In most cases, liver enzymes normalized within 1-5 months after discontinuation of ashwagandha (102686,107372,110491,111535,112111,114113). However, treatment with corticosteroids, lactulose, ornithine, ursodeoxycholic acid, and plasmapheresis, among other interventions, was required in one case (111533). Rarely, use of oral ashwagandha has been reported to cause hepatic encephalopathy, liver failure requiring liver transplantation, and acute-on-chronic liver failure resulting in death (110490,113610).
Neurologic/CNS ...Orally, ashwagandha has been reported to cause drowsiness (110492,113609). Headache, neck pain, and blurry vision have been reported in a 47-year-old female taking ashwagandha, cannabis, and venlafaxine. Imaging over the course of multiple years and hospital admissions indicated numerous instances of intracranial hemorrhage and multifocal stenosis of intracranial arteries, likely secondary to reversible cerebral vasoconstriction syndrome (RCVS) (112113). It is unclear whether the RCVS and subsequent intracranial hemorrhages were precipitated by ashwagandha, cannabis, or venlafaxine.
