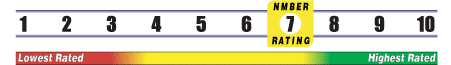
| Ingredients | Amount Per Serving |
|---|---|
|
Probiotic Blend
(50 billion CFU (colony forming units))
|
358 mg |
|
(Lactobacillus plantarum R-1012ND )
|
|
|
(Lactobacillus casei )
|
|
|
(Lactobacillus casei R0215ND )
|
|
|
(Lactobacillus rhamnosus R-11ND )
|
|
|
(Lactobacillus rhamnosus R-343ND )
|
|
|
(Lactobacillus helveticus R0052ND )
|
|
|
(Lactobacillus helveticus R0419ND )
|
|
|
(fruit)
|
90 mg |
Hypromellose, Water, Purified, Ascorbic Acid, Maltodextrin, Cellulose, Magnesium Stearate (Alt. Name: Mg Stearate)
Below is general information about the effectiveness of the known ingredients contained in the product Probiotic & Cranberry 50 Billion. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
INSUFFICIENT RELIABLE EVIDENCE to RATE
Below is general information about the safety of the known ingredients contained in the product Probiotic & Cranberry 50 Billion. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
LIKELY SAFE . .when used orally and appropriately. Cranberry juice up to 300 mL daily and cranberry extracts in doses up to 800 mg twice daily have been safely used in clinical trials (3333,3334,6758,6760,7008,8252,8253,8254,8995,11328) (16415,16720,17100,17126,17176,17210,17524,46379,46388,46389)(46390,46425,46439,46443,46465,46456,46466,46467,46469,46471)(46496,46499,90044,102847,111407).
CHILDREN: LIKELY SAFE
when cranberry juice is consumed in amounts commonly found in the diet (2811,6759,46441,46452,46470,111407).
There is insufficient reliable information available about the safety of cranberry when used in medicinal amounts in children.
PREGNANCY AND LACTATION: LIKELY SAFE
when consumed in amounts commonly found in the diet.
There is insufficient reliable information available about the safety of cranberry when used therapeutically during pregnancy or lactation; avoid using.
LIKELY SAFE ...when used orally and appropriately. Lacticaseibacillus casei has been safely used alone or in combination with other ingredients in studies lasting up to 8 weeks (90230,112517).
CHILDREN: LIKELY SAFE
when used orally and appropriately in children of most ages.
Lacticaseibacillus casei has been safely used alone in studies lasting up to 4 months (14373,107544). Also, a specific mixture (Latopic, Biomed S.A.) providing 1 billion CFUs of L. casei ŁOCK 0919 50%, Lacticaseibacillus rhamnosus ŁOCK 0900 25%, and L. rhamnosus ŁOCK 0908 25% has been used with apparent safety for 3 months in children under 2 years of age (107510). In addition, in children ages 4-17 years, a 1:1:1 combination of L. casei CECT 9104, Bifidobacterium animalis subsp. lactis CECT 8145, and Bifidobacterium longum CECT 7347 providing 1 billion CFUs has been used with apparent safety for 12 weeks (107531). There is insufficient reliable information available about the safety of L. casei in preterm infants with a birth weight under 1000 grams. Cases of bacteremia have occurred rarely in preterm infants given other probiotics (102416,111610,111612,111613,111850,111852,111853). The US Food and Drug Administration (FDA) has issued a warning about cases of serious infections caused by probiotics reported in very preterm or very low birth weight infants under 1000 grams (111610). Similarly, the American Academy of Pediatrics does not support the routine administration of probiotics to these infants due to conflicting data on safety and efficacy (111608).
PREGNANCY: POSSIBLY SAFE
when used orally and appropriately.
A combination of Lacticaseibacillus casei, Lactobacillus acidophilus, and Bifidobacterium bifidum has been used with apparent safety for 6 weeks, starting at 24-28 weeks' gestation (95416,98430).
LACTATION:
There is insufficient reliable information available about the safety of Lacticaseibacillus casei during lactation.
However, there are currently no reasons to expect safety concerns when used appropriately.
LIKELY SAFE ...when used orally and appropriately. Lactobacillus acidophilus has been safely used as part of multi-ingredient probiotic products in studies lasting up to nine months (1731,6087,14370,14371,90231,90296,92255,103438,12775,107581)(110950,110970,110979,110998,111785,111793). ...when used intravaginally and appropriately. L. acidophilus has been used safely in studies lasting up to 12 weeks (12108,13176,13177,90265). There is insufficient reliable information available about the safety of non-viable, heat-killed L. acidophilus formulations when used orally.
CHILDREN: LIKELY SAFE
when used orally and appropriately in children of most ages.
Lactobacillus acidophilus has been safely used for up to 5 days (96887). Also, combination probiotics containing L. acidophilus have been used with apparent safety in various doses and durations. L. acidophilus has been combined with Bifidobacterium animalis (HOWARU Protect, Danisco) for up to 6 months in children 3-5 years old (16847), with Bifidobacterium bifidum for 6 weeks (90602,96890), with Bifidobacterium bifidum and Bifidobacterium animalis subsp. lactis (Complete Probiotic Platinum) for 18 months in children 4 months to 5 years of age (103436), and in a specific product (Visbiome, ExeGi Pharma) containing a total of 8 species for 3 months in children 2-12 years old (107497). There is insufficient reliable information available about the safety of L. acidophilus in preterm infants with a birth weight under 1000 grams. Cases of bacteremia have occurred rarely in preterm infants given other probiotics (102416,111610,111612,111613,111850,111852,111853). The US Food and Drug Administration (FDA) has issued a warning about cases of serious infections caused by probiotics reported in very preterm or very low birth weight infants under 1000 grams (111610). Similarly, the American Academy of Pediatrics does not support the routine administration of probiotics to these infants due to conflicting data on safety and efficacy (111608).
PREGNANCY: POSSIBLY SAFE
when used orally and appropriately.
A combination of Lactobacillus acidophilus, Lacticaseibacillus casei, and Bifidobacterium bifidum has been used with apparent safety for 6 weeks, starting at 24-28 weeks' gestation (95416,98430).
LACTATION:
There is insufficient reliable information available about the safety of Lactobacillus acidophilus during lactation.
However, there are currently no reasons to expect safety concerns when used appropriately.
Below is general information about the interactions of the known ingredients contained in the product Probiotic & Cranberry 50 Billion. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
Theoretically, cranberry might increase levels and adverse effects of atorvastatin.
In one case report, a patient taking atorvastatin experienced upper back pain, rhabdomyolysis, and abnormal liver function after drinking cranberry juice 16 ounces daily for 2 weeks. Theoretically, this may have been caused by inhibition of cytochrome P450 3A4 (CYP3A4) enzymes by cranberry juice, as atorvastatin is a CYP3A4 substrate. Creatinine kinase and liver enzymes normalized within 2 weeks of stopping cranberry juice (90042). Patients taking atorvastatin should avoid large quantities of cranberry juice.
|
Theoretically, cranberry might increase the levels and adverse effects of CYP2C9 substrates. However, research is conflicting.
There is contradictory evidence about the effect of cranberry on CYP2C9 enzymes. In vitro evidence suggests that flavonoids in cranberry inhibit CYP2C9 enzymes (10452,11115,90048). However, clinical research shows that cranberry juice does not significantly affect the levels, metabolism, or elimination of the CYP2C9 substrates flurbiprofen or diclofenac (11094,90048). Also, in patients stabilized on warfarin, drinking cranberry juice 250 mL daily for 7 days does not significantly increase the anticoagulant activity of warfarin, a CYP2C9 substrate (15374). Additional pharmacokinetic research shows that cranberry juice does not increase peak plasma concentrations or area under the concentration-time curve of warfarin (15393).
|
Theoretically, cranberry might increase the levels and adverse effects of CYP3A4 substrates.
A case of upper back pain, rhabdomyolysis, and abnormal liver function has been reported for a patient taking atorvastatin, a CYP3A4 substrate, in combination with cranberry juice 16 ounces daily for 2 weeks. Creatinine kinase and liver enzymes normalized within 2 weeks of stopping cranberry juice (90042). Also, animal research suggests that cranberry juice, administered intraduodenally 30 minutes prior to nifedipine, a CYP3A4 substrate, inhibits nifedipine metabolism and increases the area under the concentration-time curve by 1.6-fold compared to control (46420).
|
Theoretically, cranberry might modestly increase the levels and adverse effects of diclofenac.
|
Theoretically, cranberry might increase the levels and adverse effects of nifedipine.
Animal research suggests that cranberry juice, administered intraduodenally 30 minutes prior to nifedipine treatment, inhibits nifedipine metabolism and increases the area under the concentration-time curve by 1.6-fold compared to control (46420). This interaction has not been reported in humans.
|
Theoretically, cranberry might increase the levels and adverse effects of warfarin. However, research is conflicting.
There is contradictory evidence about the effect of cranberry juice on warfarin. Case reports have linked cranberry juice consumption to increases in the international normalized ratio (INR) in patients taking warfarin, resulting in severe spontaneous bleeding and excessive postoperative bleeding (10452,12189,12668,21187,21188,21189,46378,46396,46411)(46415,90043). Daily consumption of cranberry sauce for one week has also been linked to an increase in INR in one case report (16816). In a small study in healthy young males, taking a high dose of 3 grams of cranberry juice concentrate capsules, equivalent to 57 grams of fruit daily, for 2 weeks produced a 30% increase in the area under the INR-time curve after a single 25-mg dose of warfarin (16416). However, 3 very small clinical studies in patients stabilized on warfarin reported that cranberry juice 250 mL once or twice daily for 7 days (27% cranberry juice or pure cranberry juice) or 240 mL once daily for 14 days does not significantly increase INR or affect plasma warfarin levels (15374,17124,90045). The reasons for these discrepant findings are unclear. It is possible that the form and dose of cranberry may play a role, as cranberry extracts and juices contain different constituents. Additionally, an in vitro study evaluating 5 different cranberry juices found varying effects, with only a cranberry concentrate, and not diluted cranberry juices, inhibiting CYP2C9. However, this concentrate did not inhibit CYP2C9 activity in humans (108062).
|
Theoretically, taking Lacticaseibacillus casei with antibiotic drugs might decrease the effectiveness of L. casei.
L. casei preparations usually contain live and active organisms. Therefore, simultaneously taking antibiotics might kill a significant number of the organisms (1740). Tell patients to separate administration of antibiotics and L. casei preparations by at least two hours.
|
Theoretically, taking Lactobacillus acidophilus with antibiotic drugs might decrease the effectiveness of L. acidophilus.
L. acidophilus preparations usually contain live and active organisms. Therefore, simultaneously taking antibiotics might kill a significant number of the organisms (1740). Tell patients to separate administration of antibiotics and L. acidophilus preparations by at least two hours.
|
Below is general information about the adverse effects of the known ingredients contained in the product Probiotic & Cranberry 50 Billion. Some ingredients may not be listed. This information does NOT represent a recommendation for or a test of this specific product as a whole.
General
...Orally, cranberry seems to be well tolerated.
Most Common Adverse Effects:
Orally: Diarrhea and gastrointestinal discomfort.
Dermatologic ...Orally, skin redness and itching has been reported in one patient (46389).
Gastrointestinal ...In very large doses, for example 3-4 L per day of juice, cranberry can cause gastrointestinal upset and diarrhea, particularly in young children (46364). There are reports of abdominal and gastrointestinal discomfort after taking cranberry tablets, extracts, and juice in clinical trials (16720,46379,111407). Nausea, vomiting, and diarrhea have also been reported with consumption of lower doses of cranberry juice cocktail, 16 ounces per day, equivalent to about 4 ounces cranberry juice, for several weeks (16415).
Genitourinary ...Vulvovaginal candidiasis has been associated with ingestion of cranberry juice (46374). Clinical research suggests that ingestion of cranberry juice may be associated with vaginal itching and vaginal dryness (46471). One patient in clinical research stopped taking dried cranberry juice due to excessive urination (46437), and an isolated case of nocturia following ingestion of cranberry tablets has been reported (16720).
Hematologic ...Thrombocytopenia has been reported as an adverse event to cranberry juice (46459).
Other ...An isolated case of sensitive swollen nipples after taking cranberry tablets has been reported (16720).
General
...Orally, Lacticaseibacillus casei is generally well tolerated.
Most Common Adverse Effects:
Orally: Mild gastrointestinal adverse effects.
Serious Adverse Effects (Rare):
Orally: There is concern that lactobacilli may cause infections in some people.
Gastrointestinal ...Orally, taking Lacticaseibacillus casei in combination with other probiotics may cause gastrointestinal side effects including abdominal pain (90291); however, these events are uncommon.
Immunologic
...Since Lacticaseibacillus casei preparations contain live and active microorganisms, there is some concern that they might cause pathogenic infection in some patients.
Some lactobacilli species have been isolated in some cases of bacteremia, sepsis, splenic abscess, endocarditis, aortic dissection, necrotizing fasciitis, pancreatic necrosis, and meningoencephalitis. Most of these cases are thought to be due to the translocation of bacteria from other locations in the body in which they occur naturally, such as the oral cavity and gastrointestinal tract. The majority of cases are not related to the use of probiotic supplements and most are not associated with the use of L. casei (107543,112516). There is at least one case of L. casei bacteremia and endocarditis thought to be related with L. casei intake in a 71-year-old immunocompromised female (112520).
There are two cases of L. casei infection in a prosthetic joint (90282,112514). In one case, the 95-year-old female with a history of hypertension, diabetes, and heart disease was known to consume yogurt containing L. casei. However, it was not confirmed that the infection was related to the consumption of this product. Spread from the gastrointestinal tract or vaginal flora could not be ruled out (90282). In the case of an 80-year-old male, the cause was unknown as there was no probiotic supplementation and no underlying medical condition or infectious portal of entry (112514).
A specific probiotic preparation (NBL probiotic ATP, Nobel) containing L. casei, Lacticaseibacillus rhamnosus, Lactiplantibacillus plantarum, Bifidobacterium animalis subsp. lactis, fructo-oligosaccharides, galacto-oligosaccharides, colostrum, and lactoferrin was found to be a significant risk factor for vancomycin-resistant Enterococcus colonization in premature infants. Although there was no direct link to determine causation, it was hypothesized that the probiotic mixture helped to mediate the acquisition and transfer of antibiotic resistance genes (96890).
General
...Orally and intravaginally, Lactobacillus acidophilus is generally well tolerated.
Most Common Adverse Effects:
Orally: Mild gastrointestinal adverse effects.
Intravaginally: Vaginal discharge.
Serious Adverse Effects (Rare):
Orally: There is concern that L. acidophilus may cause infections in some people.
Dermatologic ...Orally, in one clinical trial, a combination of Lactobacillus acidophilus La-5, Lacticaseibacillus paracasei subsp. paracasei F19, and Bifidobacterium animalis subsp. lacltis BB-12 was associated with two cases of rash, one with itching. However, it is not clear if these adverse effects were due to L. acidophilus, other ingredients, the combination, or if the events were idiosyncratic (90236).
Gastrointestinal ...Orally, taking Lactobacillus acidophilus in combination with other probiotics may cause gastrointestinal side effects including epigastric discomfort (90239), abdominal pain (90239,90291,111785), dyspepsia (90239), flatulence (107497,107520), bloating (107497,111785), diarrhea (111785), vomiting (107537), and burping (90239); however, these events are uncommon.
Genitourinary ...Intravaginally, cream containing Lactobacillus acidophilus has been shown to cause increased vaginal discharge in about 5% of patients, compared to about 1% of patients receiving placebo cream (90237). Vaginal burning was reported by one person using intravaginal L. acidophilus and Limosilactobacillus fermentum in a clinical trial (111781).
Immunologic ...Since Lactobacillus acidophilus preparations contain live and active microorganisms, there is some concern that they might cause pathogenic infection in some patients. L. acidophilus has been isolated in some cases of bacteremia, sepsis, splenic abscess, liver abscess, endocarditis, necrotizing fasciitis, pancreatic necrosis, and meningoencephalitis. Most of these cases are thought to be due to the translocation of bacteria from other locations in the body in which they occur naturally, such as the oral cavity and gastrointestinal tract (107543,111782,111792). L. acidophilus endophthalmitis has been reported rarely (111787,111795). In one case, it was related to intravitreal injections for age-related macular degeneration in a 90-year-old female with an intraocular lens (111787). In another, it occurred following cataract surgery (111795).
